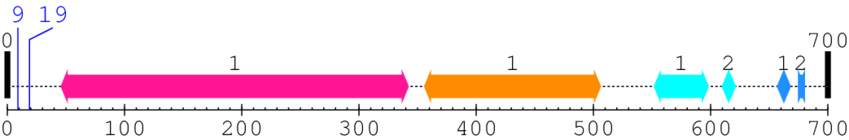SB0001 : CAPN2, m-calpain catalytic subunit, mCL
[ CaMP Format ]
* Basic Information
| Organism | Homo sapiens (human) |
| Protein Names | calpain 2, large subunit [Homo sapiens]; calpain 2, large subunit; calpain, large polypeptide L2; calcium-activated neutral proteinase; Calpain 2, large [catalytic] subunit; Calpain-2 catalytic subunit; 3.4.22.53; Calcium-activated neutral proteinase 2; CANP 2; Calpain M-type; Calpain large polypeptide L2; Calpain-2 large subunit; Millimolar-calpain; M-calpain |
| Gene Names | CAPN2; CANPL2; calpain 2, (m/II) large subunit |
| Gene Locus | 1q41-q42; chromosome 1 |
| GO Function | GO:0004198 - calpain activity [Evidence IEA]; GO:0005509 - calcium ion binding [Evidence IEA] |
* Information From OMIM
Description: The calpains, or calcium-activated neutral proteases (EC 3.4.22.17), are nonlysosomal intracellular cysteine proteases. The mammalian calpains include 2 ubiquitous isoforms, calpain I (mu-calpain) and calpain II (m-calpain), 2 stomach-specific proteins, and CAPN3 (OMIM:114240), which is muscle-specific. Calpain I and calpain II are heterodimers with distinct large subunits, encoded by the CAPN1 (OMIM:114220) and CAPN2 genes, respectively, associated with a common small subunit (CAPNS1; OMIM:114170).
* Structure Information
1. Primary Information
Length: 700 aa
Average Mass: 80.006 kDa
Monoisotopic Mass: 79.956 kDa
2. Domain Information
Annotated Domains: interpro / pfam / smart / prosite
Computationally Assigned Domains (Pfam+HMMER):
| domain name | begin | end | score | e-value |
|---|---|---|---|---|
| --- cleavage 9 --- | ||||
| --- cleavage 19 --- | ||||
| Calpain family cysteine protease 1. | 46 | 342 | 1.0 | 0.0 |
| Calpain large subunit, domain III 1. | 356 | 506 | 1.0 | 0.0 |
| EF-hand domain pair 1. | 552 | 598 | 20.0 | 0.0 |
| EF-hand domain pair 2. | 610 | 621 | 31.0 | 0.0 |
| EF hand 1. | 657 | 668 | 17.0 | 0.0 |
| EF hand 2. | 675 | 680 | 4.0 | 0.0 |
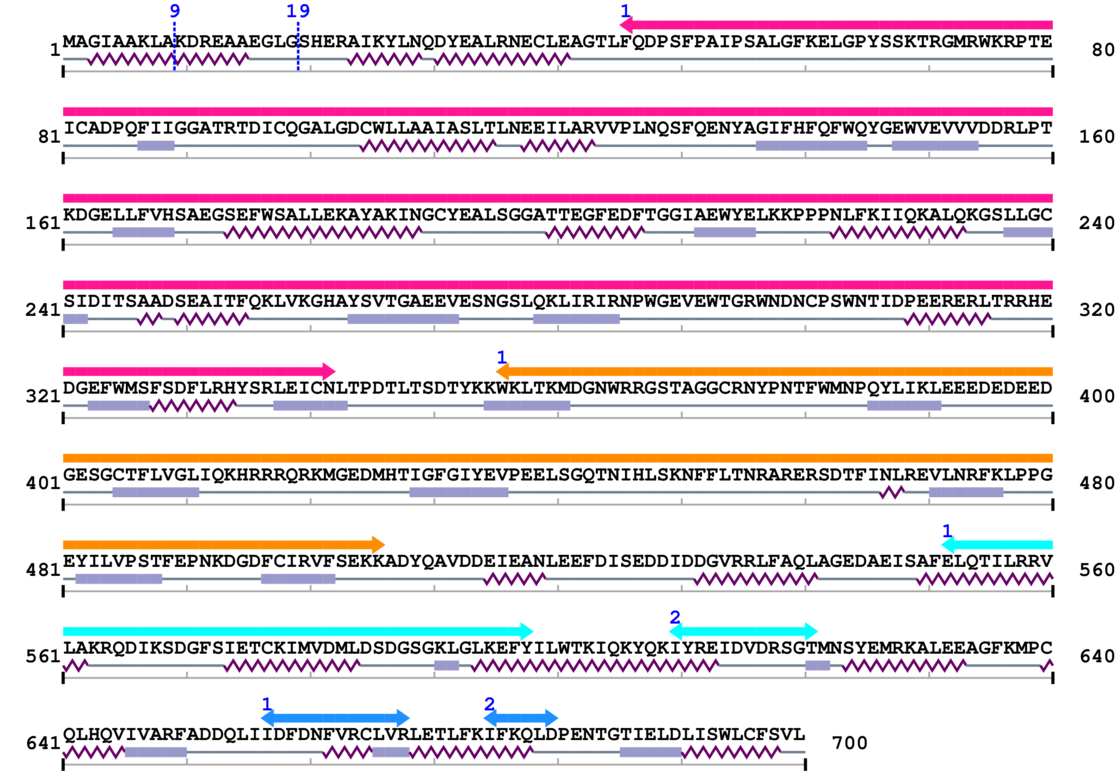
3. Sequence Information
Fasta Sequence: SB0001.fasta
Amino Acid Sequence and Secondary Structures (PsiPred):
* Cleavage Information
2 [sites] cleaved by Calpain 2
Source Reference: [PubMed ID: 8482370] Brown N, Crawford C, Structural modifications associated with the change in Ca2+ sensitivity on activation of m-calpain. FEBS Lett. 1993 May 3;322(1):65-8.
Cleavage sites (±10aa)
[Site 1] MAGIAAKLA9-KDREAAEGLG
Ala9  Lys
Lys
 |
|||||||||
| P10 | P9 | P8 | P7 | P6 | P5 | P4 | P3 | P2 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| - | Met1 | Ala2 | Gly3 | Ile4 | Ala5 | Ala6 | Lys7 | Leu8 | Ala9 |
 |
|||||||||
| P1' | P2' | P3' | P4' | P5' | P6' | P7' | P8' | P9' | P10' |
| Lys10 | Asp11 | Arg12 | Glu13 | Ala14 | Ala15 | Glu16 | Gly17 | Leu18 | Gly19 |
 Sequence conservation (by blast)
Sequence conservation (by blast)
[Site 2] KDREAAEGLG19-SHERAIKYLN
Gly19  Ser
Ser
 |
|||||||||
| P10 | P9 | P8 | P7 | P6 | P5 | P4 | P3 | P2 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| Lys10 | Asp11 | Arg12 | Glu13 | Ala14 | Ala15 | Glu16 | Gly17 | Leu18 | Gly19 |
 |
|||||||||
| P1' | P2' | P3' | P4' | P5' | P6' | P7' | P8' | P9' | P10' |
| Ser20 | His21 | Glu22 | Arg23 | Ala24 | Ile25 | Lys26 | Tyr27 | Leu28 | Asn29 |
 Sequence conservation (by blast)
Sequence conservation (by blast)
* References
[PubMed ID: 17359359] Medana IM, Day NP, Hien TT, Mai NT, Bethell D, Phu NH, Turner GD, Farrar J, White NJ, Esiri MM, Cerebral calpain in fatal falciparum malaria. Neuropathol Appl Neurobiol. 2007 Apr;33(2):179-92.