| # |
organism |
max score |
hits |
top seq |
| 1 |
Oryctolagus cuniculus |
124.00 |
1 |
phosphorylase kinase, gamma 1 (muscle) |
| 2 |
Bos taurus |
119.00 |
3 |
phosphorylase kinase gamma subunit 1 |
| 3 |
Homo sapiens |
118.00 |
9 |
phosphorylase kinase gamma subunit 1 |
| 4 |
synthetic construct |
118.00 |
2 |
phosphorylase kinase, gamma 1 (muscle) |
| 5 |
Rattus norvegicus |
117.00 |
1 |
phosphorylase kinase, gamma 1 |
| 6 |
N/A |
116.00 |
12 |
1405343A phosphorylase kinase gamma |
| 7 |
Mus musculus |
115.00 |
1 |
phosphorylase kinase gamma 1 |
| 8 |
Canis familiaris |
114.00 |
6 |
PREDICTED: similar to Phosphorylase b kinase gamm |
| 9 |
Sus scrofa |
112.00 |
1 |
phosphorylase kinase gamma subunit |
| 10 |
Xenopus laevis |
97.80 |
2 |
phosphoserine phosphatase/phosphorylase kinase, g |
| 11 |
Danio rerio |
96.30 |
5 |
hypothetical protein LOC565379 |
| 12 |
Xenopus tropicalis |
95.90 |
3 |
phosphorylase kinase gamma subunit 1 |
| 13 |
Tetraodon nigroviridis |
84.30 |
7 |
unnamed protein product |
| 14 |
Danio rerio |
70.50 |
1 |
hypothetical protein LOC393937 |
| 15 |
synthetic construct |
49.70 |
1 |
phosphorylase kinase, gamma 2 |
| 16 |
Mus musculus |
48.50 |
6 |
phosphorylase kinase, gamma 2 (testis) |
| 17 |
Rattus norvegicus |
47.80 |
5 |
phosphorylase kinase, gamma 2 (testis) |
| 18 |
Halocynthia roretzi |
45.40 |
1 |
phosphorylase kinase B gamma subunit |
| 19 |
Drosophila pseudoobscura |
35.40 |
1 |
GA18909-PA |
| 20 |
Drosophila melanogaster |
34.70 |
3 |
MAP kinase |
| 21 |
Drosophila melanogaster |
34.70 |
1 |
Mpk2 CG5475-PA, isoform A |
| 22 |
Entamoeba histolytica HM-1:IMSS |
33.50 |
1 |
protein kinase domain containing protein |
| 23 |
Cryptococcus neoformans var. neoformans JEC21 |
33.50 |
1 |
glycogen synthase kinase 3 |
| 24 |
Trypanosoma brucei TREU927 |
33.10 |
1 |
protein kinase |
| 25 |
Giardia lamblia ATCC 50803 |
32.30 |
1 |
Long-flagella protein, kinase, CMGC RCK |
| 26 |
Cyprinus carpio |
32.00 |
1 |
extracellular signal regulated protein kinase 1 |
| 27 |
Oryza sativa (japonica cultivar-group) |
31.60 |
2 |
Os12g0169800 |
| 28 |
Glossina morsitans morsitans |
31.60 |
1 |
MAP kinase activity 2 |
| 29 |
Strongylocentrotus purpuratus |
31.60 |
1 |
MAP kinase |
| 30 |
Dictyostelium discoideum AX4 |
31.60 |
1 |
extracellular response kinase |
| 31 |
Bombyx mori |
31.60 |
1 |
Extracellular regulated MAP kinase |
| 32 |
Dictyostelium discoideum AX4 |
31.20 |
1 |
extracellular signal-regulated protein kinase |
| 33 |
Homo sapiens |
31.20 |
1 |
protein serine/threonine kinase |
| 34 |
Limulus polyphemus |
30.80 |
2 |
calcium/calmodulin-dependent protein kinase type |
| 35 |
Oikopleura dioica |
30.80 |
1 |
calcium/calmodulin-dependent protein kinase type |
| 36 |
Ovis aries |
30.40 |
1 |
Ca2+/calmodulin-dependent protein kinase II |
| 37 |
Macaca mulatta |
28.50 |
1 |
AF478034_1 cyclin-dependent kinase 2 |
| organism | matching |
|---|
| Oryctolagus cuniculus |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
||||||||||||||||||||||||||||||#||||||||||||||||||||||||||||||
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
|
| Bos taurus |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
|+| +|||||||||||||||||||||||||#||||||||||||||||||||||||||||||
Sbjct 274 QRRCSAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
|
| Homo sapiens |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
| ||||||||||||||||+|||||||||||#||||| ||||||||||||||||||||||||
Sbjct 274 QNRYTAEEALAHPFFQQYLVEEVRHFSPRG#KFKVIALTVLASVRIYYQYRRVKPVTREIV 333
|
| synthetic construct |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
| ||||||||||||||||+|||||||||||#||||| ||||||||||||||||||||||||
Sbjct 274 QNRYTAEEALAHPFFQQYLVEEVRHFSPRG#KFKVIALTVLASVRIYYQYRRVKPVTREIV 333
|
| Rattus norvegicus |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
| | +|||||||||||+|||||||||||||#||||||||||||||||||||||||||||||
Sbjct 274 QDRCSAEEALAHPFFQEYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
|
| N/A |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
| | +|||||||||||+|||||||||||||#||||||||||||||||||||||||||||||
Sbjct 273 QDRCSAEEALAHPFFQEYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 332
|
| Mus musculus |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
| | +|||||||||||+|||||||||||||#|||||||||||||+||||||||||||||||
Sbjct 274 QDRCSAEEALAHPFFQEYVVEEVRHFSPRG#KFKVICLTVLASVKIYYQYRRVKPVTREIV 333
|
| Canis familiaris |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
| | +|||||||||||||||||||||||||#||||| ||||||||||||||||||||||||
Sbjct 417 QGRCSAEEALAHPFFQQYVVEEVRHFSPRG#KFKVIALTVLASVRIYYQYRRVKPVTREIV 476
|
| Sus scrofa |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
| | +|||||||||||||||||||||||||#||||| ||||||||||||||||||||||||
Sbjct 4 QSRCSAEEALAHPFFQQYVVEEVRHFSPRG#KFKVIALTVLASVRIYYQYRRVKPVTREIV 63
|
| Xenopus laevis |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
++| ||+||| ||||||| |||||+||| #||||+|||||||||||+ ||+||||||||+
Sbjct 273 ERRLTADEALVHPFFQQYDVEEVRYFSPLR#KFKVVCLTVLASVRIYHYYRKVKPVTREII 332
|
| Danio rerio |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
+||+|| +|| ||||||||| ||||||| #||||||+||||++||| ||| ||||+|++
Sbjct 274 EKRFTATDALNHPFFQQYVVAEVRHFSPYR#KFKVICMTVLATMRIYCNYRRAKPVTKEVI 333
|
| Xenopus tropicalis |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
++| ||+||| ||||||| |||||+||| #||||+|||||||||||+ ||+|||||+||+
Sbjct 273 ERRLTADEALIHPFFQQYDVEEVRYFSPFR#KFKVVCLTVLASVRIYHYYRKVKPVTKEII 332
|
| Tetraodon nigroviridis |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
++|+|| + | | || ||||+||| ||| #+|||||+||||++||| ||| ||||+|++
Sbjct 274 KQRFTATDVLNHSFFSQYVVDEVREFSPYR#RFKVICMTVLATMRIYCSYRRAKPVTKEVI 333
|
| Danio rerio |
Query 276 RYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTREIV 333
| |||+||||||| || |+|| |||| # || + ||||| +|+ +| | +|+|+|++
Sbjct 279 RLTAEQALAHPFFCQYQKEDVRLFSPRK#TFKALILTVLACIRMQCRYYRARPLTKELL 336
|
| synthetic construct |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTR 330
+ | |||+|| ||||++ + + +|| #+|+| ||||+ |+ ||+|+|+
Sbjct 277 EARLTAEQALQHPFFERCEGSQPWNLTPRQ#RFRVAVWTVLAAGRVALSTHRVRPLTK 333
|
| Mus musculus |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTR 330
+ | |||+|| ||||++ + + +|| #+|+| |+||+ |+ |++|+|+
Sbjct 277 EARLTAEQALQHPFFERCEGSQPWNLTPRQ#RFRVAVWTILAAGRVALSSHRLRPLTK 333
|
| Rattus norvegicus |
Query 276 RYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKVICLTVLASVRIYYQYRRVKPVTR 330
| |||+|| ||||++ + + +|| #+|+| |+||+ |+ |++|+|+
Sbjct 279 RLTAEQALQHPFFERCKGSQPWNLTPRQ#RFRVAVWTILAAGRVALSSHRLRPLTK 333
|
| Halocynthia roretzi |
Query 275 KRYTAEEALAHPFFQQYVVEEVRH----FSPRG#KFKVICLTVLASVRIYYQYRRVKPV 328
+| |||||| |||| | | +| |+ |+ + #||+| |||+|+ || || + |
Sbjct 272 ERITAEEALRHPFFDQ-VDQESRYYSKAFNAKR#KFRVAILTVIATSRIVRQYHNPRTV 328
|
| Drosophila pseudoobscura |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSP 301
+|| |||||||||+ ++| | ||
Sbjct 298 EKRITAEEALAHPYLEKYSEPSDEHTSP 325
|
| Drosophila melanogaster |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSP 301
+|| ||||||+||+ ++| | ||
Sbjct 298 EKRITAEEALSHPYLEKYAEPSVEQTSP 325
|
| Drosophila melanogaster |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSP 301
+|| ||||||+||+ ++| | ||
Sbjct 298 EKRITAEEALSHPYLEKYAEPSVEQTSP 325
|
| Entamoeba histolytica HM-1:IMSS |
Query 275 KRYTAEEALAHPFFQQYVVEEVRHFSPRG#KFKV 307
|| | ||||||||| +++|+ | #+ |+
Sbjct 361 KRITPREALAHPFFQSGIIKEINPLFKRE#EQKM 393
|
| Cryptococcus neoformans var. neoformans JEC21 |
Query 276 RYTAEEALAHPFFQQYVVEEVRHFSPRG#K 304
|||| ||| |||| + || | | |#|
Sbjct 317 RYTAPEALVHPFFDELRVEGAR--LPNG#K 343
|
| Trypanosoma brucei TREU927 |
Query 274 QKRYTAEEALAHPFFQQYVVEEV 296
+|| | ++|||||||+ ||| |
Sbjct 295 RKRLTVDKALAHPFFKMYVVPGV 317
|
| Giardia lamblia ATCC 50803 |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSP 301
||| |||||| ||||+ + | || +|
Sbjct 290 QKRITAEEALRHPFFKD-IGEIVRLKAP 316
|
| Cyprinus carpio |
Query 275 KRYTAEEALAHPFFQQY-------VVEEVRHFS------PRG#KFKVICLTVLASVRIYYQ 321
|| | |||||||+ +|| | || |+ |+ #| | + | + ||
Sbjct 331 KRITVEEALAHPYLEQYYDPSDEPVAEEPFTFNMELDDLPKE#KLKELIYEETARFQATYQ 390
|
| Oryza sativa (japonica cultivar-group) |
Query 275 KRYTAEEALAHPF-----------FQQYVVEEVRHFSPRG#KFKVICLTVLA 314
||+|| | | ||+ |+ ++||| #| | + | |+|
Sbjct 294 KRFTAHEVLCHPWIVDDAVAPDKPIDSAVLSRLKHFSAMN#KLKKMALRVIA 344
|
| Glossina morsitans morsitans |
Query 274 QKRYTAEEALAHPFFQQYVVEEVRHFSP 301
+|| |||+|||||+ ++| ||
Sbjct 293 EKRITAEQALAHPYMEKYFEPSDEQTSP 320
|
| Strongylocentrotus purpuratus |
Query 275 KRYTAEEALAHPFFQQY 291
|| | |||||||+ +||
Sbjct 306 KRITVEEALAHPYLEQY 322
|
| Dictyostelium discoideum AX4 |
Query 275 KRYTAEEALAHPFFQQY 291
|| |||||||||| |+
Sbjct 291 KRITAEEALAHPFVTQF 307
|
| Bombyx mori |
Query 274 QKRYTAEEALAHPFFQQY 291
|| | |||||||+ +||
Sbjct 302 HKRITVEEALAHPYLEQY 319
|
| Dictyostelium discoideum AX4 |
Query 275 KRYTAEEALAHPFFQ 289
|| | |||||||+||
Sbjct 424 KRLTVEEALAHPYFQ 438
|
| Homo sapiens |
Query 275 KRYTAEEALAHPFFQQY 291
|| | |||||||+ +||
Sbjct 317 KRITVEEALAHPYLEQY 333
|
| Limulus polyphemus |
Query 275 KRYTAEEALAHPFF-----------QQYVVEEVRHFSPRG#KFKVICLTVLASVRIY 319
|| || ||| ||+ +| || ++ |+ | #| | || + + | +
Sbjct 259 KRITASEALKHPWICQRERVASTLHRQETVECLKKFNARR#KLKGAILTTMLATRNF 314
|
| Oikopleura dioica |
Query 274 QKRYTAEEALAHPFF-----------QQYVVEEVRHFSPRG#KFKVICLTVLASVR 317
+|| |||+|| ||+ +| || ++ |+ | #| | +| + + |
Sbjct 258 KKRITAEQALNHPWISQRERYAATIHRQETVECLKKFNARR#KLKGAIITTMLATR 312
|
| Ovis aries |
Query 274 QKRYTAEEALAHPFFQ-----------QYVVEEVRHFSPRG#KFKVICLTVLASVRIY 319
|| || ||| ||+ | |+ ++ |+ | #| | || + + | +
Sbjct 35 SKRITAAEALKHPWISHRSTVASCMHRQETVDCLKKFNARR#KLKGAILTTMLATRNF 91
|
| Macaca mulatta |
Query 275 KRYTAEEALAHPFFQQYVVEEVRHF 299
|| +|+ |||||||| | + | |
Sbjct 1 KRISAKAALAHPFFQD-VTKPVPHL 24
|
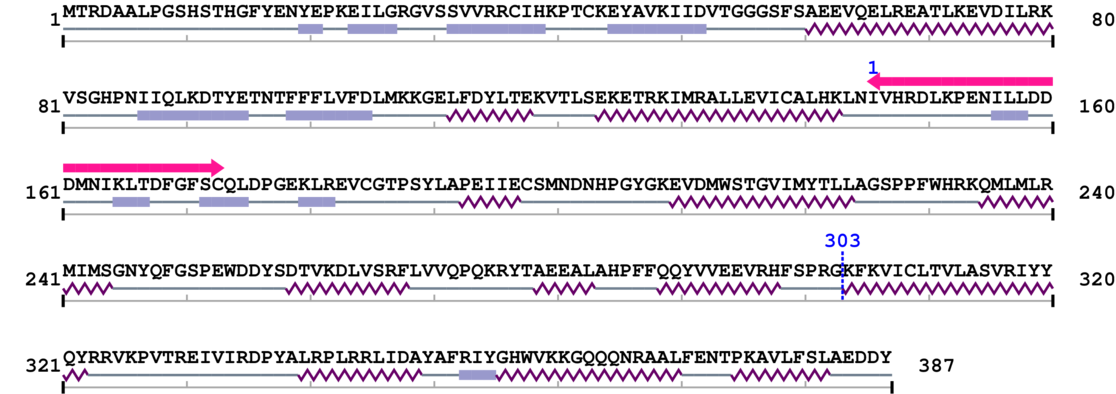
 Lys
Lys

 Sequence conservation (by blast)
Sequence conservation (by blast)