SB0031 : [beta]-Actin
[ CaMP Format ]
* Basic Information
| Organism | Homo sapiens (human) |
| Protein Names | actin, cytoplasmic 1 [Homo sapiens]; actin, cytoplasmic 1; beta cytoskeletal actin; PS1TP5-binding protein 1; Actin, cytoplasmic 1; Beta-actin; Actin, cytoplasmic 1, N-terminally processed |
| Gene Names | ACTB; actin, beta |
| Gene Locus | 7p22; chromosome 7 |
| GO Function | Not available |
* Information From OMIM
Function: Interaction of phospholipase D (see PLD1; OMIM:602382) with actin microfilaments regulates cell proliferation, vesicle trafficking, and secretion. Kusner et al. (2002) found that highly purified globular actin (G-actin) inhibited both basal and stimulated PLD1 activity, whereas filamentous actin (F-actin) had the opposite effect. Actin-induced modulation of PLD1 activity was independent of the activating stimulus. The effects of actin on PLD1 were isoform specific: human platelet actin, which exists in a 5:1 ratio of beta- and gamma-actin, was only 45% as potent and 40% as efficacious as rabbit skeletal muscle alpha-actin.
* Structure Information
1. Primary Information
Length: 375 aa
Average Mass: 41.736 kDa
Monoisotopic Mass: 41.710 kDa
2. Domain Information
Annotated Domains: interpro / pfam / smart / prosite
Computationally Assigned Domains (Pfam+HMMER):
| domain name | begin | end | score | e-value |
|---|---|---|---|---|
| Actin 1. | 3 | 375 | 2.0 | 0.0 |
| --- cleavage 37 (inside Actin 3..375) --- | ||||
3. Sequence Information
Fasta Sequence: SB0031.fasta
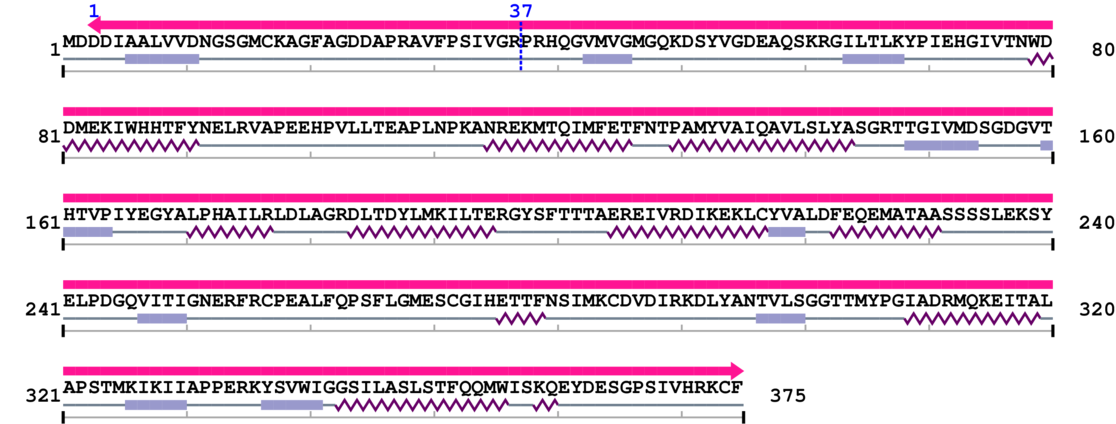
Amino Acid Sequence and Secondary Structures (PsiPred):
* Cleavage Information
1 [sites] cleaved by Calpain 1 and/or 2
Source Reference: [PubMed ID: 9472000] Villa PG, Henzel WJ, Sensenbrenner M, Henderson CE, Pettmann B, Calpain inhibitors, but not caspase inhibitors, prevent actin proteolysis and DNA fragmentation during apoptosis. J Cell Sci. 1998 Mar;111 ( Pt 6):713-22.
Cleavage sites (±10aa)
[Site 1] RAVFPSIVGR37-PRHQGVMVGM
Arg37  Pro
Pro
 |
|||||||||
| P10 | P9 | P8 | P7 | P6 | P5 | P4 | P3 | P2 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| Arg28 | Ala29 | Val30 | Phe31 | Pro32 | Ser33 | Ile34 | Val35 | Gly36 | Arg37 |
 |
|||||||||
| P1' | P2' | P3' | P4' | P5' | P6' | P7' | P8' | P9' | P10' |
| Pro38 | Arg39 | His40 | Gln41 | Gly42 | Val43 | Met44 | Val45 | Gly46 | Met47 |
 Sequence conservation (by blast)
Sequence conservation (by blast) Sequence conservation (by blast)
Sequence conservation (by blast)
| Reference peptide (cleaved bond±30 residues) |
|---|
| LVVDNGSGMCKAGFAGDDAPRAVFPSIVGRPRHQGVMVGMGQKDSYVGDEAQSKRGILTL |
Summary
| # | organism | max score | hits | top seq |
|---|---|---|---|---|
| 1 | Lymantria dispar | 131.00 | 1 | AF182715_1 actin |
| 2 | N/A | 130.00 | 24 | A Chain A, Crystal Structure Of Caenorhabditis El |
| 3 | Mus musculus | 130.00 | 8 | unnamed protein product |
| 4 | Aedes aegypti | 130.00 | 4 | actin 6 |
| 5 | Homo sapiens | 130.00 | 4 | beta actin |
| 6 | Caenorhabditis elegans | 130.00 | 4 | actin |
| 7 | Limulus polyphemus | 130.00 | 3 | ACTY_LIMPO Actin-11 gi |
| 8 | Drosophila melanogaster | 130.00 | 3 | Actin 57B CG10067-PA, isoform A |
| 9 | Brugia malayi | 130.00 | 3 | actin 1 |
| 10 | Danio rerio | 130.00 | 3 | bactin1 |
| 11 | Artemia sp. | 130.00 | 2 | ACT1_ARTSX Actin, clone 205 gi |
| 12 | Helicoverpa zea | 130.00 | 2 | ACT3A_HELAM Actin, cytoplasmic A3a gi |
| 13 | Biomphalaria glabrata | 130.00 | 2 | ACTC_BIOGL Actin, cytoplasmic gi |
| 14 | Artemia franciscana | 130.00 | 2 | actin |
| 15 | Saccoglossus kowalevskii | 130.00 | 2 | ACT1_SACKO Actin-1 gi |
| 16 | Drosophila melanogaster | 130.00 | 2 | Actin 5C CG4027-PB, isoform B |
| 17 | synthetic construct | 130.00 | 2 | actin gamma 1 |
| 18 | Xenopus laevis | 130.00 | 2 | hypothetical protein LOC398459 |
| 19 | Xenopus tropicalis | 130.00 | 2 | actin, gamma 1 |
| 20 | Pagrus major | 130.00 | 2 | B-actin |
| 21 | Bombyx mori | 130.00 | 2 | ACT3_BOMMO Actin, cytoplasmic A3 gi |
| 22 | Pneumocystis carinii | 130.00 | 2 | actin |
| 23 | Heliothis virescens | 130.00 | 1 | AF368030_1 actin |
| 24 | Platichthys flesus | 130.00 | 1 | beta-actin |
| 25 | Physalaemus pustulosus | 130.00 | 1 | beta actin |
| 26 | Pongo pygmaeus | 130.00 | 1 | hypothetical protein |
| 27 | Boophilus microplus | 130.00 | 1 | actin |
| 28 | Branchiostoma belcheri | 130.00 | 1 | ACTC_BRABE Actin, cytoplasmic (BbCA1) gi |
| 29 | Panagrellus redivivus | 130.00 | 1 | actin |
| 30 | Biomphalaria tenagophila | 130.00 | 1 | ACTC_BIOTE Actin, cytoplasmic gi |
| 31 | Branchiostoma floridae | 130.00 | 1 | ACTC_BRAFL Actin, cytoplasmic (BfCA1) gi |
| 32 | Oryctolagus cuniculus | 130.00 | 1 | gamma non-muscle actin |
| 33 | Ornithodoros moubata | 130.00 | 1 | actin |
| 34 | Oreochromis mossambicus | 130.00 | 1 | ACTB1_FUGRU Actin, cytoplasmic 1 (Beta-actin A) g |
| 35 | Branchiostoma lanceolatum | 130.00 | 1 | ACTC_BRALA Actin, cytoplasmic gi |
| 36 | Oikopleura longicauda | 130.00 | 1 | cytoplasmic actin |
| 37 | Rana lessonae | 130.00 | 1 | ACTG_RANLE Actin, cytoplasmic 2 (Gamma-actin) (Cy |
| 38 | Biomphalaria pfeifferi | 130.00 | 1 | ACTC_BIOPF Actin, cytoplasmic gi |
| 39 | Morulius calbasu | 130.00 | 1 | AF393832_1 beta-actin |
| 40 | Monosiga brevicollis | 130.00 | 1 | actin |
| 41 | Monopterus albus | 130.00 | 1 | beta-actin |
| 42 | Rhipicephalus appendiculatus | 130.00 | 1 | actin |
| 43 | Mayetiola destructor | 130.00 | 1 | ACT_MAYDE Actin gi |
| 44 | Biomphalaria obstructa | 130.00 | 1 | ACTC_BIOOB Actin, cytoplasmic gi |
| 45 | Lumbricus terrestris | 130.00 | 1 | ACT2_LUMTE Actin-2 gi |
| 46 | Litopenaeus vannamei | 130.00 | 1 | AF300705_1 beta-actin |
| 47 | Salmo salar | 130.00 | 1 | ACTB_SALSA Actin, cytoplasmic 1 (Beta-actin) gi |
| 48 | Lethenteron japonicum | 130.00 | 1 | cytoplasmic actin |
| 49 | Ixodes ricinus | 130.00 | 1 | actin |
| 50 | Hydroides elegans | 130.00 | 1 | Actin |
| 51 | Setaria digitata | 130.00 | 1 | actin |
| 52 | Homo sapiens | 130.00 | 1 | mutant beta-actin (beta'-actin) |
| 53 | Heterodera glycines | 130.00 | 1 | AF318603_1 actin 1 |
| 54 | Helisoma trivolvis | 130.00 | 1 | ACTC_HELTI Actin, cytoplasmic gi |
| 55 | Plectus acuminatus | 130.00 | 1 | actin |
| 56 | Sigmodon hispidus | 130.00 | 1 | ACTB_SIGHI Actin, cytoplasmic 1 (Beta-actin) gi |
| 57 | Caenorhabditis elegans | 130.00 | 1 | ACTin family member (act-4) |
| 58 | Haemaphysalis longicornis | 130.00 | 1 | actin |
| 59 | Caenorhabditis briggsae AF16 | 130.00 | 1 | hypothetical protein CBG18256 |
| 60 | Globodera rostochiensis | 130.00 | 1 | actin 2 |
| 61 | Gallus gallus | 130.00 | 1 | beta-actin |
| 62 | Gallus gallus | 130.00 | 1 | actin, gamma 1 propeptide |
| 63 | Echinococcus granulosus | 130.00 | 1 | ACT2_ECHGR Actin-2 gi |
| 64 | Strongylocentrotus purpuratus | 130.00 | 1 | PREDICTED: similar to cytoskeletal actin |
| 65 | Aplysia californica | 130.00 | 1 | ACTM_APLCA Actin, muscle gi |
| 66 | Carassius auratus | 130.00 | 1 | beta actin |
| 67 | Apis mellifera | 130.00 | 1 | PREDICTED: similar to Actin-5C isoform 1 |
| 68 | Dicentrarchus labrax | 130.00 | 1 | beta actin |
| 69 | Daphnia magna | 130.00 | 1 | actin |
| 70 | Triakis scyllium | 130.00 | 1 | ACTG_TRISC Actin, cytoplasmic 2 (Gamma-actin) gi |
| 71 | Culex pipiens pipiens | 130.00 | 1 | actin |
| 72 | Cricetinae gen. sp. | 130.00 | 1 | beta actin |
| 73 | Chrysophrys auratus | 130.00 | 1 | beta-actin |
| 74 | Tigriopus japonicus | 129.00 | 2 | AF466280_1 beta-actin |
| 75 | Tetraodon nigroviridis | 129.00 | 2 | unnamed protein product |
| 76 | Seriola quinqueradiata | 129.00 | 1 | beta-actin |
| 77 | Cryptococcus neoformans var. neoformans JEC21 | 129.00 | 1 | actin |
| 78 | Chlamys farreri | 129.00 | 1 | actin |
| 79 | Xenopus borealis | 129.00 | 1 | ACTB_XENBO Actin, cytoplasmic 1 (Beta actin) gi |
| 80 | Crassostrea gigas | 129.00 | 1 | Actin 2 |
| 81 | Cirrhinus molitorella | 129.00 | 1 | beta-actin |
| 82 | Suillus bovinus | 129.00 | 1 | ACT1_SUIBO Actin-1 gi |
| 83 | Ciona intestinalis | 129.00 | 1 | act protein |
| 84 | Halocynthia roretzi | 129.00 | 1 | ACTC_HALRO Actin, nonmuscle gi |
| 85 | Biomphalaria alexandrina | 129.00 | 1 | ACTC_BIOAL Actin, cytoplasmic gi |
| 86 | Molgula oculata | 129.00 | 1 | cytoskeletal actin 1 |
| 87 | Oncorhynchus mykiss | 129.00 | 1 | AF157514_1 beta-actin |
| 88 | Oryzias latipes | 129.00 | 1 | cytoplasmic actin OlCA1 |
| 89 | Reticulitermes flavipes | 129.00 | 1 | actin |
| 90 | Bos taurus | 129.00 | 1 | beta-actin |
| 91 | Rhynchocypris oxycephalus | 129.00 | 1 | beta-actin |
| 92 | Phanerochaete chrysosporium | 129.00 | 1 | actin |
| 93 | Passer domesticus | 129.00 | 1 | AF416454_1 beta-actin |
| 94 | Pseudopleuronectes americanus | 125.00 | 2 | beta actin |
| 95 | Absidia glauca | 125.00 | 2 | ACT1_ABSGL Actin-1 gi |
| 96 | Canis familiaris | 125.00 | 1 | beta-actin |
| 97 | Paxillus involutus | 125.00 | 1 | AF457916_1 putative actin |
| 98 | Dugesia polychroa | 124.00 | 3 | actin 1 |
| 99 | Gryllus bimaculatus | 124.00 | 1 | actin |
| 100 | Bubalus bubalis | 124.00 | 1 | beta-actin |
| 101 | Cavia porcellus | 124.00 | 1 | AF191277_1 cytoplasmic actin |
| 102 | Drosophila americana | 123.00 | 1 | actin E2 |
| 103 | Salvelinus alpinus | 123.00 | 1 | beta-actin |
Top-ranked sequences
| organism | matching |
|---|---|
| Lymantria dispar | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| N/A | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Mus musculus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Aedes aegypti | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Homo sapiens | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Caenorhabditis elegans | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Limulus polyphemus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Drosophila melanogaster | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Brugia malayi | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Danio rerio | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Artemia sp. | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Helicoverpa zea | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Biomphalaria glabrata | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Artemia franciscana | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 9 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 68 |
| Saccoglossus kowalevskii | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||+|||||||#|||||||||||||||||||||||||||||| Sbjct 9 LVVDNGSGMCKAGFAGDDAPRAIFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 68 |
| Drosophila melanogaster | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| synthetic construct | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 8 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Xenopus laevis | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Xenopus tropicalis | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Pagrus major | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Bombyx mori | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Pneumocystis carinii | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||+|||||||||||||||||||||||| Sbjct 8 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGIMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Heliothis virescens | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Platichthys flesus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Physalaemus pustulosus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 10 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 69 |
| Pongo pygmaeus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Boophilus microplus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Branchiostoma belcheri | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Panagrellus redivivus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Biomphalaria tenagophila | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Branchiostoma floridae | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Oryctolagus cuniculus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Ornithodoros moubata | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Oreochromis mossambicus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Branchiostoma lanceolatum | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Oikopleura longicauda | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Rana lessonae | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 8 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Biomphalaria pfeifferi | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Morulius calbasu | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Monosiga brevicollis | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Monopterus albus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Rhipicephalus appendiculatus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Mayetiola destructor | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Biomphalaria obstructa | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Lumbricus terrestris | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Litopenaeus vannamei | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Salmo salar | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Lethenteron japonicum | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Ixodes ricinus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Hydroides elegans | Query 9 VVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 10 VIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 68 |
| Setaria digitata | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Homo sapiens | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Heterodera glycines | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Helisoma trivolvis | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Plectus acuminatus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 9 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 68 |
| Sigmodon hispidus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Caenorhabditis elegans | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Haemaphysalis longicornis | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Caenorhabditis briggsae AF16 | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Globodera rostochiensis | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Gallus gallus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Gallus gallus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Echinococcus granulosus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 9 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 68 |
| Strongylocentrotus purpuratus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Aplysia californica | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Carassius auratus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Apis mellifera | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Dicentrarchus labrax | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Daphnia magna | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Triakis scyllium | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Culex pipiens pipiens | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Cricetinae gen. sp. | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Chrysophrys auratus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Tigriopus japonicus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Tetraodon nigroviridis | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Seriola quinqueradiata | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Cryptococcus neoformans var. neoformans JEC21 | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 10 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 69 |
| Chlamys farreri | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Xenopus borealis | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 9 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 68 |
| Crassostrea gigas | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Cirrhinus molitorella | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Suillus bovinus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 8 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Ciona intestinalis | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Halocynthia roretzi | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Biomphalaria alexandrina | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Molgula oculata | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Oncorhynchus mykiss | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Oryzias latipes | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Reticulitermes flavipes | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Bos taurus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Rhynchocypris oxycephalus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Phanerochaete chrysosporium | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 1 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 60 |
| Passer domesticus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Pseudopleuronectes americanus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Absidia glauca | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||+|||||||||||||||||||||||| Sbjct 10 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGIMVGMGQKDSYVGDEAQSKRGILTL 69 |
| Canis familiaris | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Paxillus involutus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 8 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Dugesia polychroa | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Gryllus bimaculatus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 |
| Bubalus bubalis | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 6 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 65 |
| Cavia porcellus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#||||||||||||||||||||||||||+||| Sbjct 8 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGVLTL 67 |
| Drosophila americana | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||+|||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 9 LVIDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 68 |
| Salvelinus alpinus | Query 8 LVVDNGSGMCKAGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 67 ||||||||||| ||||||||||||||||||#|||||||||||||||||||||||||||||| Sbjct 6 LVVDNGSGMCKTGFAGDDAPRAVFPSIVGR#PRHQGVMVGMGQKDSYVGDEAQSKRGILTL 65 |
* References
[PubMed ID: 24415753] Sobierajska K, Skurzynski S, Stasiak M, Kryczka J, Cierniewski CS, Swiatkowska M, Protein disulfide isomerase directly interacts with beta-actin Cys374 and regulates cytoskeleton reorganization. J Biol Chem. 2014 Feb 28;289(9):5758-73. doi: 10.1074/jbc.M113.479477. Epub 2014
[PubMed ID: 24129186] ... Shiokawa N, Nakamura M, Sameshima M, Deguchi A, Hayashi T, Sasaki N, Sano A, Chorein, the protein responsible for chorea-acanthocytosis, interacts with beta-adducin and beta-actin. Biochem Biophys Res Commun. 2013 Nov 8;441(1):96-101. doi:
[PubMed ID: 23535377] ... Lui YL, Lin Z, Lee JJ, Chow VT, Poh CL, Tan EL, Beta-actin variant is necessary for Enterovirus 71 replication. Biochem Biophys Res Commun. 2013 Apr 19;433(4):607-10. doi:
[PubMed ID: 23466368] ... Thompson PM, Tolbert CE, Campbell SL, Vinculin and metavinculin: oligomerization and interactions with F-actin. FEBS Lett. 2013 Apr 17;587(8):1220-9. doi: 10.1016/j.febslet.2013.02.042. Epub
[PubMed ID: 24068947] ... Brandler WM, Morris AP, Evans DM, Scerri TS, Kemp JP, Timpson NJ, St Pourcain B, Smith GD, Ring SM, Stein J, Monaco AP, Talcott JB, Fisher SE, Webber C, Paracchini S, Common variants in left/right asymmetry genes and pathways are associated with relative hand skill. PLoS Genet. 2013;9(9):e1003751. doi: 10.1371/journal.pgen.1003751. Epub 2013 Sep
[PubMed ID: 18083107] ... Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ, Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007 Dec 14;131(6):1190-203.
[PubMed ID: 15592455] ... Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ, Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol. 2005 Jan;23(1):94-101. Epub 2004 Dec 12.
[PubMed ID: 11955010] ... Nyman T, Schuler H, Korenbaum E, Schutt CE, Karlsson R, Lindberg U, The role of MeH73 in actin polymerization and ATP hydrolysis. J Mol Biol. 2002 Apr 5;317(4):577-89.
[PubMed ID: 10866806] ... Schuler H, Nyakern M, Schutt CE, Lindberg U, Karlsson R, Mutational analysis of arginine 177 in the nucleotide binding site of beta-actin. Eur J Biochem. 2000 Jul;267(13):4054-62.
[PubMed ID: 9209005] ... Shartava A, Korn W, Shah AK, Goodman SR, Irreversibly sickled cell beta-actin: defective filament formation. Am J Hematol. 1997 Jun;55(2):97-103.
[PubMed ID: 8605189] ... Bencsath FA, Shartava A, Monteiro CA, Goodman SR, Identification of the disulfide-linked peptide in irreversibly sickled cell beta-actin. Biochemistry. 1996 Apr 9;35(14):4403-8.
[PubMed ID: 8619402] ... Shartava A, Miranda P, Williams KN, Shah A, Monteiro CA, Goodman SR, High density sickle cell erythrocyte core membrane skeletons demonstrate slow temperature dependent dissociation. Am J Hematol. 1996 Mar;51(3):214-9.
[PubMed ID: 7876306] ... Shartava A, Monteiro CA, Bencsath FA, Schneider K, Chait BT, Gussio R, Casoria-Scott LA, Shah AK, Heuerman CA, Goodman SR, A posttranslational modification of beta-actin contributes to the slow dissociation of the spectrin-protein 4.1-actin complex of irreversibly sickled cells. J Cell Biol. 1995 Mar;128(5):805-18.
[PubMed ID: 1629950] ... Horisberger MA, Interferon-induced human protein MxA is a GTPase which binds transiently to cellular proteins. J Virol. 1992 Aug;66(8):4705-9.
[PubMed ID: 1555776] ... Harris DE, Warshaw DM, Periasamy M, Nucleotide sequences of the rabbit alpha-smooth-muscle and beta-non-muscle actin mRNAs. Gene. 1992 Mar 15;112(2):265-6.
[PubMed ID: 1505215] ... Habets GG, van der Kammen RA, Willemsen V, Balemans M, Wiegant J, Collard JG, Sublocalization of an invasion-inducing locus and other genes on human chromosome 7. Cytogenet Cell Genet. 1992;60(3-4):200-5.
[PubMed ID: 3335520] ... Vandekerckhove J, Schering B, Barmann M, Aktories K, Botulinum C2 toxin ADP-ribosylates cytoplasmic beta/gamma-actin in arginine 177. J Biol Chem. 1988 Jan 15;263(2):696-700.
[PubMed ID: 435297] ... Smith SS, Kelly KH, Jockusch BM, Actin co-purifies with RNA polymerase II. Biochem Biophys Res Commun. 1979 Jan 15;86(1):161-6.
[PubMed ID: 274701] ... Vandekerckhove J, Weber K, Mammalian cytoplasmic actins are the products of at least two genes and differ in primary structure in at least 25 identified positions from skeletal muscle actins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1106-10.