SB0137 : Protein Phosphatase 2A (PP2A) Regulatory Subunit [alpha]4, immunoglobulin-binding protein 1
[ CaMP Format ]
* Basic Information
| Organism | Homo sapiens (human) |
| Protein Names | immunoglobulin-binding protein 1 [Homo sapiens]; immunoglobulin-binding protein 1; protein phosphatase 2A, regulatory subunit alpha-4; B cell signal transduction molecule alpha 4; bA351K23.1 (immunoglobulin binding protein 1 (CD79A)); protein alpha-4; CD79a-binding protein 1; renal carcinoma antigen NY-REN-16; B-cell signal transduction molecule alpha 4; protein phosphatase 2/4/6 regulatory subunit; Immunoglobulin-binding protein 1; Protein alpha-4; Protein phosphatase 2/4/6 regulatory subunit; Renal carcinoma antigen NY-REN-16 |
| Gene Names | IGBP1; IBP1; immunoglobulin (CD79A) binding protein 1 |
| Gene Locus | Xq13.1-q13.3; chromosome X |
| GO Function | Not available |
* Information From OMIM
Function: Wildtype Mid1 (OMIM:300552) colocalizes predominantly with microtubules, in contrast to mutant versions of Mid1 found in Opitz syndrome (OMIM:300000) patients that appear clustered in the cytosol. Using yeast 2-hybrid screening, Liu et al. (2001) found that the alpha-4 subunit of protein phosphatases-2A, -4, and -6 bound Mid1. Localization of Mid1 and the alpha-4 subunit was influenced by one another in transiently transfected cells. Mid1 could recruit the alpha-4 subunit onto microtubules, and high levels of the alpha-4 subunit could displace Mid1 into the cytosol. Metabolic (32)P labeling of cells revealed Mid1 to be a phosphoprotein, and coexpression of the full-length alpha-4 subunit decreased Mid1 phosphorylation, indicative of a functional interaction. Association of GFP-Mid1 with microtubules in living cells was perturbed by inhibitors of MAP kinase activation. Liu et al. (2001) concluded that Mid1 association with microtubules, which seems important for normal midline development, is regulated by dynamic phosphorylation involving MAP kinase and protein phosphatase that is targeted specifically to Mid1 by the alpha-4 subunit.
* Structure Information
1. Primary Information
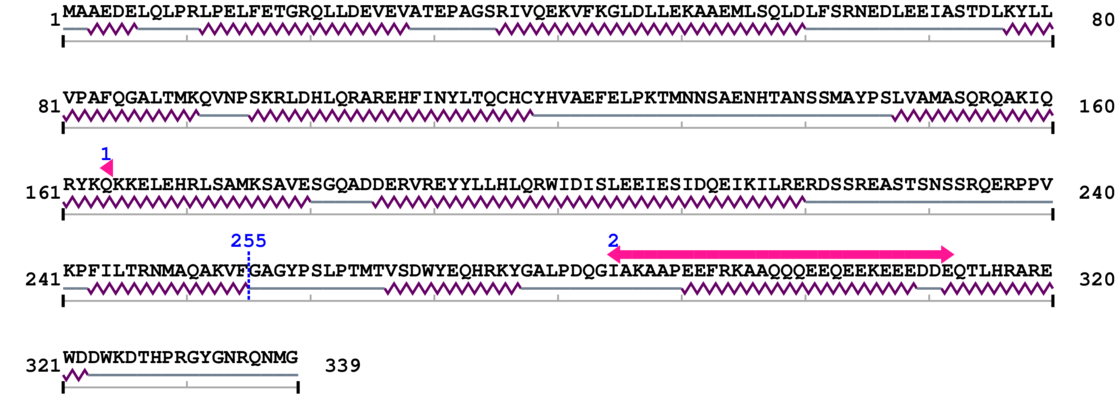
Length: 339 aa
Average Mass: 39.221 kDa
Monoisotopic Mass: 39.198 kDa
2. Domain Information
Annotated Domains: interpro / pfam / smart / prosite
Computationally Assigned Domains (Pfam+HMMER):
| domain name | begin | end | score | e-value |
|---|---|---|---|---|
| 60s Acidic ribosomal protein 1. | 164 | 164 | 70.0 | 0.3 |
| --- cleavage 255 --- | ||||
| 60s Acidic ribosomal protein 2. | 285 | 312 | 53.0 | 1.8 |
3. Sequence Information
Fasta Sequence: SB0137.fasta
Amino Acid Sequence and Secondary Structures (PsiPred):
4. 3D Information
Known Structures in PDB: 4IYP (X-ray; 280 A; A=2-234)
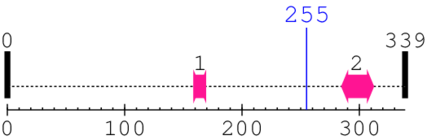
* Cleavage Information
1 [sites] cleaved by Calpain ?
Source Reference: [PubMed ID: 22613722] Watkins GR, Wang N, Mazalouskas MD, Gomez RJ, Guthrie CR, Kraemer BC, Schweiger S, Spiller BW, Wadzinski BE, Monoubiquitination promotes calpain cleavage of the protein phosphatase 2A (PP2A) regulatory subunit alpha4, altering PP2A stability and microtubule-associated protein phosphorylation. J Biol Chem. 2012 Jul 13;287(29):24207-15. doi: 10.1074/jbc.M112.368613. Epub
Cleavage sites (±10aa)
[Site 1] TRNMAQAKVF255-GAGYPSLPTM
Phe255  Gly
Gly
 |
|||||||||
| P10 | P9 | P8 | P7 | P6 | P5 | P4 | P3 | P2 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| Thr246 | Arg247 | Asn248 | Met249 | Ala250 | Gln251 | Ala252 | Lys253 | Val254 | Phe255 |
 |
|||||||||
| P1' | P2' | P3' | P4' | P5' | P6' | P7' | P8' | P9' | P10' |
| Gly256 | Ala257 | Gly258 | Tyr259 | Pro260 | Ser261 | Leu262 | Pro263 | Thr264 | Met265 |
 Sequence conservation (by blast)
Sequence conservation (by blast)
* References
[PubMed ID: 24388773] Liu J, Cai M, Chen J, Liao Y, Mai S, Li Y, Huang X, Liu Y, Zhang J, Kung H, Zeng Y, Zhou F, Xie D, alpha4 contributes to bladder urothelial carcinoma cell invasion and/or metastasis via regulation of E-cadherin and is a predictor of outcome in bladder urothelial carcinoma patients. Eur J Cancer. 2014 Mar;50(4):840-51. doi: 10.1016/j.ejca.2013.11.038. Epub 2014