SB0149 : Calpastatin
[ CaMP Format ]
* Basic Information
| Organism | Homo sapiens (human) |
| Protein Names | calpastatin isoform m [Homo sapiens]; calpastatin isoform m; calpain inhibitor; sperm BS-17 component |
| Gene Names | CAST; calpastatin |
| Gene Locus | 5q15; chromosome 5 |
| GO Function | Not available |
| Entrez Protein | Entrez Nucleotide | Entrez Gene | UniProt | OMIM | HGNC | HPRD | KEGG |
|---|---|---|---|---|---|---|---|
| NP_001177371 | NM_001190442 | 831 | N/A | 114090 | HGNC:1515 | N/A | N/A |
* Information From OMIM
Description: Calpastatin is an endogenous inhibitor of calpains (see OMIM:114170) consisting of 4 inhibitory repeats, each of which neutralizes an activated calpain. Unlike proteinases, it is an intrinsically unstructured protein, adopting a defined structure only upon binding to active calpain (Moldoveanu et al., 2008; Hanna et al., 2008).
Function: In erythrocytes of patients with essential hypertension (see OMIM:145500), the level of calpastatin activity is significantly lower than in the red cells of normotensive subjects. Pontremoli et al. (1988) demonstrated by Western blot analysis that the decreased inhibitor activity is the result of a decrease in the amount of the inhibitor protein. Calpastatin isolated and purified from erythrocytes of normotensive and hypertensive patients had identical specific activities. Pontremoli et al. (1988) also presented evidence indicating that the decreased level of calpastatin cannot be ascribed to accelerated decay during the red cell life span.
* Structure Information
1. Primary Information
Length: 695 aa
Average Mass: 75.304 kDa
Monoisotopic Mass: 75.259 kDa
2. Domain Information
Annotated Domains: Not Available.
Computationally Assigned Domains (Pfam+HMMER):
| domain name | begin | end | score | e-value |
|---|---|---|---|---|
| Calpain inhibitor 1. | 32 | 87 | 22.0 | 16.2 |
| --- cleavage 195 --- | ||||
| --- cleavage 203 --- | ||||
| --- cleavage 209 --- | ||||
| --- cleavage 210 --- | ||||
| Maf N-terminal region 1. | 213 | 224 | 23.0 | 0.0 |
| --- cleavage 228 --- | ||||
| --- cleavage 253 --- | ||||
| --- cleavage 256 --- | ||||
| Maf N-terminal region 2. | 417 | 424 | 25.0 | 0.1 |
| SlyX 1. | 554 | 603 | 15.0 | 0.7 |
| Calpain inhibitor 2. | 625 | 678 | 53.0 | 5.1 |
3. Sequence Information
Fasta Sequence: SB0149.fasta
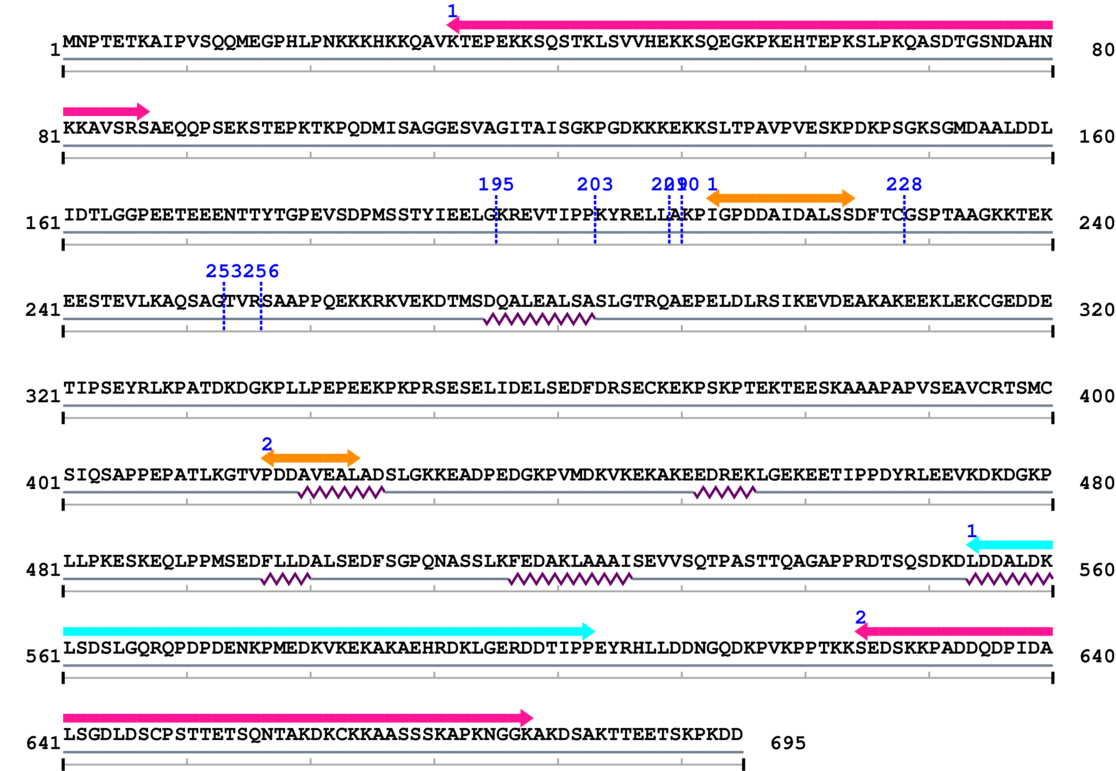
Amino Acid Sequence and Secondary Structures (PsiPred):
4. 3D Information
Not Available.
* Cleavage Information
7 [sites] cleaved by Calpain 3
Source Reference: [PubMed ID: 14594950] Ono Y, Kakinuma K, Torii F, Irie A, Nakagawa K, Labeit S, Abe K, Suzuki K, Sorimachi H, Possible regulation of the conventional calpain system by skeletal muscle-specific calpain, p94/calpain 3. J Biol Chem. 2004 Jan 23;279(4):2761-71. Epub 2003 Nov 1.
Cleavage sites (±10aa)
[Site 1] MSSTYIEELG195-KREVTIPPKY
Gly195  Lys
Lys
 |
|||||||||
| P10 | P9 | P8 | P7 | P6 | P5 | P4 | P3 | P2 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| Met186 | Ser187 | Ser188 | Thr189 | Tyr190 | Ile191 | Glu192 | Glu193 | Leu194 | Gly195 |
 |
|||||||||
| P1' | P2' | P3' | P4' | P5' | P6' | P7' | P8' | P9' | P10' |
| Lys196 | Arg197 | Glu198 | Val199 | Thr200 | Ile201 | Pro202 | Pro203 | Lys204 | Tyr205 |
 Sequence conservation (by blast)
Sequence conservation (by blast) Sequence conservation (by blast)
Sequence conservation (by blast)
| Reference peptide (cleaved bond±30 residues) |
|---|
| GPEETEEENTTYTGPEVSDPMSSTYIEELGKREVTIPPKYRELLAKPIGPDDAIDALSSD |
Summary
| # | organism | max score | hits | top seq |
|---|---|---|---|---|
| 1 | N/A | 122.00 | 9 | calpastatin |
| 2 | Homo sapiens | 114.00 | 10 | calpastatin |
| 3 | Canis familiaris | 107.00 | 3 | PREDICTED: similar to calpastatin isoform a isofo |
| 4 | Ovis aries | 100.00 | 1 | calpastatin |
| 5 | Mus musculus | 66.20 | 12 | calpastatin |
| 6 | Rattus norvegicus | 57.80 | 7 | calpastatin |
| 7 | Xenopus laevis | 50.80 | 1 | putative calpastatin |
| 8 | Xenopus laevis | 35.80 | 2 | calpastatin, putative |
| 9 | Bos taurus | 34.70 | 6 | calpastatin |
| 10 | Oryctolagus cuniculus | 34.30 | 1 | calpastatin |
| 11 | Bos taurus | 34.30 | 1 | calpastatin isoform III |
| 12 | Rattus norvegicus | 33.50 | 1 | calpastatin isoform c |
| 13 | Rattus sp. | 32.70 | 1 | calpastatin/CANP inhibitor |
| 14 | Homo sapiens | 32.00 | 2 | calpastatin isoform i |
| 15 | Pongo pygmaeus | 32.00 | 2 | hypothetical protein |
| 16 | Sus scrofa | 31.60 | 3 | calpastatin |
Top-ranked sequences
| organism | matching |
|---|---|
| N/A | Query 166 GPEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLAKPIGPDDAIDALSSD 225 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 166 GPEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLAKPIGPDDAIDALSSD 225 |
| Homo sapiens | Query 166 GPEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLA-------------KP 212 ||||||||||||||||||||||||||||||#||||||||||||||| || Sbjct 166 GPEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLAKKEGITGPPADSSKP 225 Query 213 IGPDDAIDALSSD 225 ||||||||||||| Query 213 IGPDDAIDALSSD 225 |
| Canis familiaris | Query 167 PEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLAKPIGPDDAIDALSSD 225 ||||+|+| |||||+||||+||||||||#|||||+|||||||||||+||+||||||||| Sbjct 212 PEETKEDNVPYTGPEISDPMTSTYIEELG#KREVTLPPKYRELLAKPMGPNDAIDALSSD 270 |
| Ovis aries | Query 167 PEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELL-------------AKPI 213 ||||+|+ |||||||||||||||||||||#|||||+|||||||| +||+ Sbjct 169 PEETKEDTTTYTGPEVSDPMSSTYIEELG#KREVTLPPKYRELLNKEEGIAGPPPDSSKPL 228 Query 214 GPDDAIDALSSD 225 ||+||||||||| Sbjct 229 GPNDAIDALSSD 240 |
| Mus musculus | Query 166 GPEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLA-------------KP 212 | |+| ++ |||| | ||| |||+| ||# +| ||||+||+|| || Sbjct 145 GHEDTNRDDPPYTGPVVLDPMDSTYLEALG#IKEGTIPPEYRKLLEKNEGITQPLPDSPKP 204 Query 213 IGPDDAIDALSSD 225 +| | |||||||| Sbjct 205 MGTDQAIDALSSD 217 |
| Rattus norvegicus | Query 168 EETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLA-------------KPIG 214 |+| +++ |||| | ||| |||+| ||# +| ||||+||+|| ||+| Sbjct 109 EDTNKDDPPYTGPVVLDPMDSTYLEALG#IKEETIPPEYRKLLEKNEAITGPLPDSPKPMG 168 Query 215 PDDAIDA 221 | |||| Sbjct 169 IDHAIDA 175 |
| Xenopus laevis | Query 166 GPEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELL 209 ||+ | |+ |||||| + +| | ||||#||| +||| || || Sbjct 77 GPDVTVPESPVYTGPEVMETSTSEYREELG#KREGSIPPAYRHLL 120 |
| Xenopus laevis | Query 166 GPEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELL 209 || |+ + ||||++ + |||+||||#||+ ||||+|| || Sbjct 39 GPPANVPESPVFEGPEVAETIKSTYLEELG#KRDHTIPPEYRNLL 82 Query 166 GPEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELL 209 ||+ | |+ |+||||++ ++ | ||||#||+ +||| || || Sbjct 177 GPDLTVPESPVYSGPEVTETSTAVYREELG#KRDDSIPPAYRHLL 220 Query 182 VSDPMSSTYIEELG#KREVTIPPKYRELL--------------------AKPIGPDDAIDA 221 | + + +|+ ||# |+ ||||+||+|| ||+ | |||| Sbjct 469 VKEKAKAEHIDRLG#DRDDTIPPEYRKLLDGKDDKGQAAKPPVKEEEKPKKPLSDDAAIDA 528 Query 222 LSS 224 ||| Query 222 LSS 224 |
| Bos taurus | Query 181 EVSDPMSSTYIEELG#KREVTIPPKYRELL--------------------AKPIGPDDAID 220 +| + + + ++||#+|+ ||||||+ || || | | || Sbjct 53 KVKEKAKAEHRDKLG#ERDDTIPPKYQHLLDDNKEGTPGKPKASEKPKASEKPAGAQDPID 112 Query 221 ALSSD 225 ||| | Sbjct 113 ALSGD 117 |
| Oryctolagus cuniculus | Query 167 PEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLAK-------------PI 213 | ||+|++| |||||+|||||||||||||#|||||||||||||| | |+ Sbjct 167 PSETQEDSTAYTGPEISDPMSSTYIEELG#KREVTIPPKYRELLEKKTGVAGPPPDSVTPL 226 Query 214 GPDDAIDALSSD 225 |||||||||||| Query 214 GPDDAIDALSSD 225 Query 190 YIEELG#KREVTIPPKYRELL-------------------AKPIGPDDAIDALSSD 225 + ++||#+|+ ||||+|| || || | | ||||| | Sbjct 607 HKDKLG#ERDDTIPPEYRHLLDQGEQDKPEKPPTKKSKEIKKPAGDQDPIDALSGD 661 |
| Bos taurus | Query 167 PEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLA-------------KPI 213 ||| +|+||||||||||||||||||||||#||| |+||||+||| ||+ Sbjct 169 PEEMKEDNTTYTGPEVSDPMSSTYIEELG#KRESTLPPKYKELLNKEEGIAGPPPDSLKPL 228 Query 214 GPDDAIDALSSD 225 ||+||||||||| Sbjct 229 GPNDAIDALSSD 240 Query 181 EVSDPMSSTYIEELG#KREVTIPPKYRELL--------------------AKPIGPDDAID 220 +| + + + ++||#+|+ ||||||+ || || | | || Sbjct 598 KVKEKAKAEHRDKLG#ERDDTIPPKYQHLLDDNKEGTPGKPKASEKPKASEKPAGAQDPID 657 Query 221 ALSSD 225 ||| | Sbjct 658 ALSGD 662 |
| Rattus norvegicus | Query 168 EETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLA-------------KPIG 214 |+| +++ |||| | ||| |||+| ||# +| ||||+||+|| ||+| Sbjct 145 EDTNKDDPPYTGPVVLDPMDSTYLEALG#IKEGTIPPEYRKLLEKNEAITGPLPDSPKPMG 204 Query 215 PDDAIDALSSD 225 | |||||||| Sbjct 205 IDHAIDALSSD 215 Query 181 EVSDPMSSTYIEELG#KREVTIPPKYRELL----------------AKPIGPDDAIDALSS 224 +| + + + + |+||#+|+ ||||+|| || || | ||||| Sbjct 538 KVKEKIKAEHSEKLG#ERDDTIPPEYRHLLDNDGKDKPEKPLTKNTEKPGQDQDPIDALSE 597 Query 225 D 225 | Query 225 D 225 |
| Rattus sp. | Query 168 EETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLA-------------KPIG 214 |+| +++ |||| | ||| |||+| ||# +| ||||+||+|| ||+| Sbjct 96 EDTNKDDPPYTGPVVLDPMDSTYLEALG#IKEGTIPPEYRKLLEKNEAITGPLPDSPKPMG 155 Query 215 PDDAIDALSSD 225 | |||||||| Sbjct 156 IDHAIDALSSD 166 Query 181 EVSDPMSSTYIEELG#KREVTIPPKYRELL--------AKPI-------GPD-DAIDALSS 224 +| + + + + |+||#+|+ ||||+|| || ||+ | | | ||||| Sbjct 489 KVKEKIKAEHSEKLG#ERDDTIPPEYRHLLDNDGKDKPEKPLDKEHREAGQDQDPIDALSE 548 Query 225 D 225 | Query 225 D 225 |
| Homo sapiens | Query 166 GPEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLA-------------KP 212 ||||||||||||||||||||||||||||||#||||||||||||||| || Sbjct 166 GPEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLAKKEGITGPPADSSKP 225 Query 213 IGPDDAIDALSSD 225 ||||||||||||| Query 213 IGPDDAIDALSSD 225 Query 181 EVSDPMSSTYIEELG#KREVTIPPKYRELL-----AKPIGP--------------DDAIDA 221 +| + + + ++||#+|+ ||||+|| || ||+ | | ||| Sbjct 594 KVKEKAKAEHRDKLG#ERDDTIPPEYRHLLDDNGQDKPVKPPTKKSEDSKKPADDQDPIDA 653 Query 222 LSSD 225 || | Sbjct 654 LSGD 657 |
| Pongo pygmaeus | Query 166 GPEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELLA-------------KP 212 ||||||||||||| ||||||||||||||||#+|||||||||||||| || Sbjct 152 GPEETEEENTTYTVPEVSDPMSSTYIEELG#EREVTIPPKYRELLAKNEGITGPPPDSSKP 211 Query 213 IGPDDAIDALSSD 225 +|||||||||||| Sbjct 212 MGPDDAIDALSSD 224 Query 181 EVSDPMSSTYIEELG#KREVTIPPKYRELL-----AKPIGP--------------DDAIDA 221 +| + + + ++||#+|+ ||||+|| || ||+ | | ||| Sbjct 579 KVKEKAKAEHRDKLG#ERDDTIPPEYRHLLDDNGQDKPVKPPTKKSEDSKKPADDQDPIDA 638 Query 222 LSSD 225 || | Sbjct 639 LSGD 642 |
| Sus scrofa | Query 166 GPEETEEENTTYTGPEVSDPMSSTYIEELG#KREVTIPPKYRELL-------------AKP 212 |||||||+||||||||| ||||||||||||#|||||+|||||||| +|| Sbjct 235 GPEETEEDNTTYTGPEVLDPMSSTYIEELG#KREVTLPPKYRELLDKKEGIPVPPPDTSKP 294 Query 213 IGPDDAIDALSSD 225 +|||||||||| | Sbjct 295 LGPDDAIDALSLD 307 Query 181 EVSDPMSSTYIEELG#KREVTIPPKYRELL-------------------AKPIGPDDAIDA 221 +| + + + ++||#+|+ ||||+|| || || | ||| Sbjct 662 KVKEKAEAEHRDKLG#ERDDTIPPEYRHLLDKDEEGKSTKPPTKKPEAPKKPEAAQDPIDA 721 Query 222 LSSD 225 || | Sbjct 722 LSGD 725 |
[Site 2] LGKREVTIPP203-KYRELLAKPI
Pro203  Lys
Lys
 |
|||||||||
| P10 | P9 | P8 | P7 | P6 | P5 | P4 | P3 | P2 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| Leu194 | Gly195 | Lys196 | Arg197 | Glu198 | Val199 | Thr200 | Ile201 | Pro202 | Pro203 |
 |
|||||||||
| P1' | P2' | P3' | P4' | P5' | P6' | P7' | P8' | P9' | P10' |
| Lys204 | Tyr205 | Arg206 | Glu207 | Leu208 | Leu209 | Ala210 | Lys211 | Pro212 | Ile213 |
 Sequence conservation (by blast)
Sequence conservation (by blast) Sequence conservation (by blast)
Sequence conservation (by blast)
| Reference peptide (cleaved bond±30 residues) |
|---|
| NTTYTGPEVSDPMSSTYIEELGKREVTIPPKYRELLAKPIGPDDAIDALSSDFTCGSPTA |
Summary
| # | organism | max score | hits | top seq |
|---|---|---|---|---|
| 1 | N/A | 126.00 | 9 | calpastatin |
| 2 | Homo sapiens | 119.00 | 9 | calpastatin |
| 3 | Mus musculus | 76.60 | 12 | calpastatin |
| 4 | Rattus norvegicus | 55.50 | 7 | calpastatin |
| 5 | Xenopus laevis | 48.90 | 1 | putative calpastatin |
| 6 | Bos taurus | 40.80 | 8 | calpastatin |
| 7 | Xenopus laevis | 38.50 | 2 | calpastatin, putative |
| 8 | Canis familiaris | 37.70 | 4 | PREDICTED: similar to calpastatin isoform a isofo |
| 9 | Rattus sp. | 36.60 | 1 | calpastatin/CANP inhibitor |
| 10 | Oryctolagus cuniculus | 36.60 | 1 | calpastatin |
| 11 | Sus scrofa | 35.80 | 3 | calpastatin |
| 12 | Pongo pygmaeus | 33.90 | 2 | hypothetical protein |
| 13 | Homo sapiens | 33.90 | 2 | calpastatin isoform i |
| 14 | Ovis aries | 33.50 | 1 | calpastatin |
| 15 | Bos taurus | 32.30 | 1 | calpastatin isoform III |
| 16 | Rattus norvegicus | 30.80 | 1 | calpastatin isoform c |
Top-ranked sequences
| organism | matching |
|---|---|
| N/A | Query 174 NTTYTGPEVSDPMSSTYIEELGKREVTIPP#KYRELLAKPIGPDDAIDALSSDFTCGSPTA 233 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 174 NTTYTGPEVSDPMSSTYIEELGKREVTIPP#KYRELLAKPIGPDDAIDALSSDFTCGSPTA 233 |
| Homo sapiens | Query 174 NTTYTGPEVSDPMSSTYIEELGKREVTIPP#KYRELLA-------------KPIGPDDAID 220 ||||||||||||||||||||||||||||||#||||||| |||||||||| Sbjct 174 NTTYTGPEVSDPMSSTYIEELGKREVTIPP#KYRELLAKKEGITGPPADSSKPIGPDDAID 233 Query 221 ALSSDFTCGSPTA 233 ||||||||||||| Query 221 ALSSDFTCGSPTA 233 |
| Mus musculus | Query 177 YTGPEVSDPMSSTYIEELGKREVTIPP#KYRELLA-------------KPIGPDDAIDALS 223 |||| | ||| |||+| || +| ||||#+||+|| ||+| | |||||| Sbjct 156 YTGPVVLDPMDSTYLEALGIKEGTIPP#EYRKLLEKNEGITQPLPDSPKPMGTDQAIDALS 215 Query 224 SDFTCGSPTA 233 ||||| ||| Sbjct 216 SDFTCSSPTG 225 |
| Rattus norvegicus | Query 177 YTGPEVSDPMSSTYIEELGKREVTIPP#KYRELLA-------------KPIGPDDAIDA 221 |||| | ||| |||+| || +| ||||#+||+|| ||+| | |||| Sbjct 118 YTGPVVLDPMDSTYLEALGIKEETIPP#EYRKLLEKNEAITGPLPDSPKPMGIDHAIDA 175 |
| Xenopus laevis | Query 177 YTGPEVSDPMSSTYIEELGKREVTIPP#KYRELL---------------AKPIGPDDAIDA 221 |||||| + +| | ||||||| +|||# || || +|+ | || Sbjct 88 YTGPEVMETSTSEYREELGKREGSIPP#AYRHLLDGKVDGKVAPPPPPEEEPMTDDQLIDE 147 Query 222 LSSDFTC 228 | ||+| Sbjct 148 FSMDFSC 154 |
| Bos taurus | Query 214 GPDDAIDALSSDFTCGSPTA 233 ||+|||||||||||| |||| Sbjct 1 GPNDAIDALSSDFTCSSPTA 20 |
| Xenopus laevis | Query 177 YTGPEVSDPMSSTYIEELGKREVTIPP#KYRELL-----------AKPIGPD------DAI 219 + ||||++ + |||+||||||+ ||||#+|| || + |+ |+ | + Sbjct 50 FEGPEVAETIKSTYLEELGKRDHTIPP#EYRNLLDGKDHEKAVQPSAPVEPEPTMTDADLV 109 Query 220 DALSSDF--TCGSPT 232 | | || +| ||| Sbjct 110 DEFSKDFELSC-SPT 123 Query 177 YTGPEVSDPMSSTYIEELGKREVTIPP#KYRELL---------------AKPIGPDDAIDA 221 |+||||++ ++ | ||||||+ +|||# || || +|+ | +| Sbjct 188 YSGPEVTETSTAVYREELGKRDDSIPP#AYRHLLDGKEDGKVAPPPPPEEEPLTDDQLLDE 247 Query 222 LSSDFTC 228 | ||+| Sbjct 248 FSMDFSC 254 Query 182 VSDPMSSTYIEELGKREVTIPP#KYRELL--------------------AKPIGPDDAIDA 221 | + + +|+ || |+ ||||#+||+|| ||+ | |||| Sbjct 469 VKEKAKAEHIDRLGDRDDTIPP#EYRKLLDGKDDKGQAAKPPVKEEEKPKKPLSDDAAIDA 528 Query 222 LSSDFTC 228 ||| | Sbjct 529 LSSGFAS 535 |
| Canis familiaris | Query 182 VSDPMSSTYIEELGKREVTIPP#KYRELL------------------AKPIGPDDAIDALS 223 | + + + ++||+|+ ||||#+|| || || | ||||| Sbjct 174 VKERAKAEHRDKLGERDDTIPP#EYRHLLDNDEGKPGKPPAKKTKDSKKPEDDKDPIDALS 233 Query 224 SDF-TCGSPT 232 || +| +|| Sbjct 234 GDFDSCPAPT 243 |
| Rattus sp. | Query 177 YTGPEVSDPMSSTYIEELGKREVTIPP#KYRELLA-------------KPIGPDDAIDALS 223 |||| | ||| |||+| || +| ||||#+||+|| ||+| | |||||| Sbjct 105 YTGPVVLDPMDSTYLEALGIKEGTIPP#EYRKLLEKNEAITGPLPDSPKPMGIDHAIDALS 164 Query 224 SDFTCGSPT 232 ||||| ||| Sbjct 165 SDFTCSSPT 173 Query 182 VSDPMSSTYIEELGKREVTIPP#KYRELL--------AKPI-------GPD-DAIDALSSD 225 | + + + + |+||+|+ ||||#+|| || ||+ | | | ||||| | Sbjct 490 VKEKIKAEHSEKLGERDDTIPP#EYRHLLDNDGKDKPEKPLDKEHREAGQDQDPIDALSED 549 Query 226 FTCGSPT 232 || Sbjct 550 LDSCPPT 556 |
| Oryctolagus cuniculus | Query 174 NTTYTGPEVSDPMSSTYIEELGKREVTIPP#KYRELLAK-------------PIGPDDAID 220 +| |||||+|||||||||||||||||||||#|||||| | |+||||||| Sbjct 174 STAYTGPEISDPMSSTYIEELGKREVTIPP#KYRELLEKKTGVAGPPPDSVTPLGPDDAID 233 Query 221 ALSSDFTCGSPTA 233 |||||||| || | Sbjct 234 ALSSDFTCSSPVA 246 Query 181 EVSDPMSSTYIEELGKREVTIPP#KYRELL-------------------AKPIGPDDAIDA 221 +| + + ++||+|+ ||||#+|| || || | | ||| Sbjct 598 KVKERAKKEHKDKLGERDDTIPP#EYRHLLDQGEQDKPEKPPTKKSKEIKKPAGDQDPIDA 657 Query 222 LSSDFTCGSPTA 233 || | | | Sbjct 658 LSGDLDSCPPAA 669 |
| Sus scrofa | Query 174 NTTYTGPEVSDPMSSTYIEELGKREVTIPP#KYRELL-------------AKPIGPDDAID 220 ||||||||| |||||||||||||||||+||#|||||| +||+||||||| Sbjct 243 NTTYTGPEVLDPMSSTYIEELGKREVTLPP#KYRELLDKKEGIPVPPPDTSKPLGPDDAID 302 Query 221 ALSSDFTCGSPTA 233 ||| | || |||| Sbjct 303 ALSLDLTCSSPTA 315 Query 181 EVSDPMSSTYIEELGKREVTIPP#KYRELL-------------------AKPIGPDDAIDA 221 +| + + + ++||+|+ ||||#+|| || || | ||| Sbjct 662 KVKEKAEAEHRDKLGERDDTIPP#EYRHLLDKDEEGKSTKPPTKKPEAPKKPEAAQDPIDA 721 Query 222 LSSDF 226 || || Sbjct 722 LSGDF 726 |
| Pongo pygmaeus | Query 180 PEVSDPMSSTYIEELGKREVTIPP#KYRELLA-------------KPIGPDDAIDALSSDF 226 ||||||||||||||||+|||||||#||||||| ||+||||||||||||| Sbjct 166 PEVSDPMSSTYIEELGEREVTIPP#KYRELLAKNEGITGPPPDSSKPMGPDDAIDALSSDF 225 Query 227 TCGSPTA 233 ||||||| Query 227 TCGSPTA 233 Query 182 VSDPMSSTYIEELGKREVTIPP#KYRELL-----AKPIGP--------------DDAIDAL 222 | + + + ++||+|+ ||||#+|| || ||+ | | |||| Sbjct 580 VKEKAKAEHRDKLGERDDTIPP#EYRHLLDDNGQDKPVKPPTKKSEDSKKPADDQDPIDAL 639 Query 223 SSDF-TCGSPT 232 | | +| | | Sbjct 640 SGDLDSCPSTT 650 |
| Homo sapiens | Query 174 NTTYTGPEVSDPMSSTYIEELGKREVTIPP#KYRELLA-------------KPIGPDDAID 220 ||||||||||||||||||||||||||||||#||||||| |||||||||| Sbjct 174 NTTYTGPEVSDPMSSTYIEELGKREVTIPP#KYRELLAKKEGITGPPADSSKPIGPDDAID 233 Query 221 ALSSDFTCGSPTA 233 ||||||||||||| Query 221 ALSSDFTCGSPTA 233 Query 182 VSDPMSSTYIEELGKREVTIPP#KYRELL-----AKPIGP--------------DDAIDAL 222 | + + + ++||+|+ ||||#+|| || ||+ | | |||| Sbjct 595 VKEKAKAEHRDKLGERDDTIPP#EYRHLLDDNGQDKPVKPPTKKSEDSKKPADDQDPIDAL 654 Query 223 SSDF-TCGSPT 232 | | +| | | Sbjct 655 SGDLDSCPSTT 665 |
| Ovis aries | Query 175 TTYTGPEVSDPMSSTYIEELGKREVTIPP#KYRELL-------------AKPIGPDDAIDA 221 ||||||||||||||||||||||||||+||#|||||| +||+||+||||| Sbjct 177 TTYTGPEVSDPMSSTYIEELGKREVTLPP#KYRELLNKEEGIAGPPPDSSKPLGPNDAIDA 236 Query 222 LSSDFTCGSPTA 233 ||||||| |||| Sbjct 237 LSSDFTCSSPTA 248 Query 192 EELGKREVTIPP#KYRELLAK-----------------PIGPDDAIDALSSDFTCGSPTA 233 |+||+|| ||||# || || |+ | +|||| ||| | |+ Sbjct 471 EKLGEREETIPP#DYRLEEAKDKDGKPLPPKEVKEPLPPLSEDFLLDALSKDFTVPSDTS 529 |
| Bos taurus | Query 174 NTTYTGPEVSDPMSSTYIEELGKREVTIPP#KYRELLA-------------KPIGPDDAID 220 ||||||||||||||||||||||||| |+||#||+||| ||+||+|||| Sbjct 176 NTTYTGPEVSDPMSSTYIEELGKRESTLPP#KYKELLNKEEGIAGPPPDSLKPLGPNDAID 235 Query 221 ALSSDFTCGSPTA 233 |||||||| |||| Sbjct 236 ALSSDFTCSSPTA 248 Query 182 VSDPMSSTYIEELGKREVTIPP#KYRELL--------------------AKPIGPDDAIDA 221 | + + + ++||+|+ ||||#||+ || || | | ||| Sbjct 599 VKEKAKAEHRDKLGERDDTIPP#KYQHLLDDNKEGTPGKPKASEKPKASEKPAGAQDPIDA 658 Query 222 LSSDF-TCGSPT 232 || || +| | | Sbjct 659 LSGDFDSCPSTT 670 Query 192 EELGKREVTIPP#KYRELLAK-----------------PIGPDDAIDALSSDFTCGSPTA 233 |+||++| ||||# || || |+ | +|||| ||| | |+ Sbjct 471 EKLGEKEETIPP#DYRLEEAKDKDGKPLLPKEVKEPLPPLSEDVLLDALSKDFTVPSDTS 529 |
| Rattus norvegicus | Query 177 YTGPEVSDPMSSTYIEELGKREVTIPP#KYRELLA-------------KPIGPDDAIDALS 223 |||| | ||| |||+| || +| ||||#+||+|| ||+| | |||||| Sbjct 154 YTGPVVLDPMDSTYLEALGIKEGTIPP#EYRKLLEKNEAITGPLPDSPKPMGIDHAIDALS 213 Query 224 SDFTCGSPT 232 ||||| ||| Sbjct 214 SDFTCSSPT 222 Query 182 VSDPMSSTYIEELGKREVTIPP#KYRELL----------------AKPIGPDDAIDALSSD 225 | + + + + |+||+|+ ||||#+|| || || | ||||| | Sbjct 539 VKEKIKAEHSEKLGERDDTIPP#EYRHLLDNDGKDKPEKPLTKNTEKPGQDQDPIDALSED 598 Query 226 FTCGSPT 232 || Sbjct 599 LDSCPPT 605 Query 192 EELGKREVTIPP#KYR-ELL----AKPIGPDDA------------IDALSSDFTC 228 |+||++| ||||# || |++ ||+ | +| +|||| ||+ Sbjct 412 EKLGEKEETIPP#DYRLEIVKDKDGKPLLPKEAEEQLPPLSDDFLLDALSQDFSS 465 |
[Site 3] TIPPKYRELL209-AKPIGPDDAI
Leu209  Ala
Ala
 |
|||||||||
| P10 | P9 | P8 | P7 | P6 | P5 | P4 | P3 | P2 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| Thr200 | Ile201 | Pro202 | Pro203 | Lys204 | Tyr205 | Arg206 | Glu207 | Leu208 | Leu209 |
 |
|||||||||
| P1' | P2' | P3' | P4' | P5' | P6' | P7' | P8' | P9' | P10' |
| Ala210 | Lys211 | Pro212 | Ile213 | Gly214 | Pro215 | Asp216 | Asp217 | Ala218 | Ile219 |
 Sequence conservation (by blast)
Sequence conservation (by blast) Sequence conservation (by blast)
Sequence conservation (by blast)
| Reference peptide (cleaved bond±30 residues) |
|---|
| PEVSDPMSSTYIEELGKREVTIPPKYRELLAKPIGPDDAIDALSSDFTCGSPTAAGKKTE |
Summary
| # | organism | max score | hits | top seq |
|---|---|---|---|---|
| 1 | N/A | 124.00 | 9 | calpastatin |
| 2 | Homo sapiens | 116.00 | 9 | calpastatin |
| 3 | Mus musculus | 71.20 | 12 | calpastatin |
| 4 | Rattus norvegicus | 48.50 | 7 | calpastatin |
| 5 | Bos taurus | 47.40 | 8 | calpastatin |
| 6 | Xenopus laevis | 42.70 | 1 | putative calpastatin |
| 7 | Canis familiaris | 40.00 | 4 | PREDICTED: similar to calpastatin isoform c isofo |
| 8 | Xenopus laevis | 38.10 | 2 | calpastatin, putative |
| 9 | Rattus norvegicus | 37.00 | 1 | calpastatin isoform c |
| 10 | Oryctolagus cuniculus | 36.60 | 1 | calpastatin |
| 11 | Rattus sp. | 36.20 | 1 | calpastatin/CANP inhibitor |
| 12 | Sus scrofa | 35.40 | 3 | calpastatin |
| 13 | Pongo pygmaeus | 35.00 | 2 | hypothetical protein |
| 14 | Homo sapiens | 35.00 | 2 | calpastatin isoform i |
| 15 | Ovis aries | 34.30 | 2 | calpastatin |
| 16 | Bos taurus | 33.10 | 1 | calpastatin isoform III |
Top-ranked sequences
| organism | matching |
|---|---|
| N/A | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#AKPIGPDDAIDALSSDFTCGSPTAAGKKTE 239 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#AKPIGPDDAIDALSSDFTCGSPTAAGKKTE 239 |
| Homo sapiens | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#A-------------KPIGPDDAIDALSSDF 226 ||||||||||||||||||||||||||||||#| |||||||||||||||| Sbjct 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#AKKEGITGPPADSSKPIGPDDAIDALSSDF 239 Query 227 TCGSPTAAGKKTE 239 ||||||||||||| Query 227 TCGSPTAAGKKTE 239 |
| Mus musculus | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#A-------------KPIGPDDAIDALSSDF 226 | | ||| |||+| || +| ||||+||+||# ||+| | ||||||||| Sbjct 159 PVVLDPMDSTYLEALGIKEGTIPPEYRKLL#EKNEGITQPLPDSPKPMGTDQAIDALSSDF 218 Query 227 TCGSPTAAGKKTE 239 || ||| ||++| Sbjct 219 TCSSPT--GKQSE 229 |
| Rattus norvegicus | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#A-------------KPIGPDDAIDA 221 | | ||| |||+| || +| ||||+||+||# ||+| | |||| Sbjct 121 PVVLDPMDSTYLEALGIKEETIPPEYRKLL#EKNEAITGPLPDSPKPMGIDHAIDA 175 |
| Bos taurus | Query 214 GPDDAIDALSSDFTCGSPTAAGKKTE 239 ||+|||||||||||| |||| |||| Sbjct 1 GPNDAIDALSSDFTCSSPTADAKKTE 26 |
| Xenopus laevis | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL---------------#AKPIGPDDAIDALSS 224 ||| + +| | ||||||| +||| || || # +|+ | || | Sbjct 91 PEVMETSTSEYREELGKREGSIPPAYRHLLDGKVDGKVAPPPPPE#EEPMTDDQLIDEFSM 150 Query 225 DFTC 228 ||+| Sbjct 151 DFSC 154 |
| Canis familiaris | Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL------------------#AKPIGPDDAIDALS 223 | + + + ++||+|+ ||||+|| || # || | ||||| Sbjct 338 VKERAKAEHRDKLGERDDTIPPEYRHLLDNDEGKPGKPPAKKTKDS#KKPEDDKDPIDALS 397 Query 224 SDF-TCGSPTAAGKKT 238 || +| +|| | + | Sbjct 398 GDFDSCPAPTEASENT 413 |
| Xenopus laevis | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL-----------#AKPIGPD------DAIDAL 222 |||++ + |||+||||||+ ||||+|| || #+ |+ |+ | +| Sbjct 53 PEVAETIKSTYLEELGKRDHTIPPEYRNLLDGKDHEKAVQP#SAPVEPEPTMTDADLVDEF 112 Query 223 SSDF--TCGSPT 232 | || +| ||| Sbjct 113 SKDFELSC-SPT 123 Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL---------------#AKPIGPDDAIDALSS 224 |||++ ++ | ||||||+ +||| || || # +|+ | +| | Sbjct 191 PEVTETSTAVYREELGKRDDSIPPAYRHLLDGKEDGKVAPPPPPE#EEPLTDDQLLDEFSM 250 Query 225 DFTC 228 ||+| Sbjct 251 DFSC 254 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL--------------------#AKPIGPDDAIDA 221 | + + +|+ || |+ ||||+||+|| # ||+ | |||| Sbjct 469 VKEKAKAEHIDRLGDRDDTIPPEYRKLLDGKDDKGQAAKPPVKEEEKP#KKPLSDDAAIDA 528 Query 222 LSSDF 226 ||| | Sbjct 529 LSSGF 533 |
| Rattus norvegicus | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#A-------------KPIGPDDAIDALSSDF 226 | | ||| |||+| || +| ||||+||+||# ||+| | ||||||||| Sbjct 157 PVVLDPMDSTYLEALGIKEGTIPPEYRKLL#EKNEAITGPLPDSPKPMGIDHAIDALSSDF 216 Query 227 TCGSPTAAGKKTE 239 || ||| ||+|| Sbjct 217 TCSSPT--GKQTE 227 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL----------------#AKPIGPDDAIDALSSD 225 | + + + + |+||+|+ ||||+|| || # || | ||||| | Sbjct 539 VKEKIKAEHSEKLGERDDTIPPEYRHLLDNDGKDKPEKPLTKNT#EKPGQDQDPIDALSED 598 Query 226 FTCGSPT 232 || Sbjct 599 LDSCPPT 605 |
| Oryctolagus cuniculus | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#AK-------------PIGPDDAIDALSSDF 226 ||+|||||||||||||||||||||||||||# | |+||||||||||||| Sbjct 180 PEISDPMSSTYIEELGKREVTIPPKYRELL#EKKTGVAGPPPDSVTPLGPDDAIDALSSDF 239 Query 227 TCGSPTAAGK 236 || || |+|| Sbjct 240 TCSSPVASGK 249 Query 190 YIEELGKREVTIPPKYRELL-------------------#AKPIGPDDAIDALSSDFTCGS 230 + ++||+|+ ||||+|| || # || | | ||||| | Sbjct 607 HKDKLGERDDTIPPEYRHLLDQGEQDKPEKPPTKKSKEI#KKPAGDQDPIDALSGDLDSCP 666 Query 231 PTA 233 | | Sbjct 667 PAA 669 |
| Rattus sp. | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#A-------------KPIGPDDAIDALSSDF 226 | | ||| |||+| || +| ||||+||+||# ||+| | ||||||||| Sbjct 108 PVVLDPMDSTYLEALGIKEGTIPPEYRKLL#EKNEAITGPLPDSPKPMGIDHAIDALSSDF 167 Query 227 TCGSPTAAGKKTE 239 || ||| ||+|| Sbjct 168 TCSSPT--GKQTE 178 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL--------#AKPI-------GPD-DAIDALSSD 225 | + + + + |+||+|+ ||||+|| || # ||+ | | | ||||| | Sbjct 490 VKEKIKAEHSEKLGERDDTIPPEYRHLLDNDGKDKP#EKPLDKEHREAGQDQDPIDALSED 549 Query 226 FTCGSPT 232 || Sbjct 550 LDSCPPT 556 |
| Sus scrofa | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL-------------#AKPIGPDDAIDALSSDF 226 ||| |||||||||||||||||+|||||||| #+||+|||||||||| | Sbjct 249 PEVLDPMSSTYIEELGKREVTLPPKYRELLDKKEGIPVPPPDT#SKPLGPDDAIDALSLDL 308 Query 227 TCGSPTAAGKKTE 239 || |||| ||||| Sbjct 309 TCSSPTADGKKTE 321 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL-------------------#AKPIGPDDAIDAL 222 | + + + ++||+|+ ||||+|| || # || | |||| Sbjct 663 VKEKAEAEHRDKLGERDDTIPPEYRHLLDKDEEGKSTKPPTKKPEAP#KKPEAAQDPIDAL 722 Query 223 SSDF 226 | || Sbjct 723 SGDF 726 |
| Pongo pygmaeus | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#A-------------KPIGPDDAIDALSSDF 226 ||||||||||||||||+|||||||||||||#| ||+||||||||||||| Sbjct 166 PEVSDPMSSTYIEELGEREVTIPPKYRELL#AKNEGITGPPPDSSKPMGPDDAIDALSSDF 225 Query 227 TCGSPTAAGKKTE 239 ||||||||||||| Query 227 TCGSPTAAGKKTE 239 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL-----#AKPIGP--------------DDAIDAL 222 | + + + ++||+|+ ||||+|| || # ||+ | | |||| Sbjct 580 VKEKAKAEHRDKLGERDDTIPPEYRHLLDDNGQ#DKPVKPPTKKSEDSKKPADDQDPIDAL 639 Query 223 SSDF-TCGSPTAAGKKT 238 | | +| | | + | Sbjct 640 SGDLDSCPSTTETSQNT 656 |
| Homo sapiens | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#A-------------KPIGPDDAIDALSSDF 226 ||||||||||||||||||||||||||||||#| |||||||||||||||| Sbjct 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#AKKEGITGPPADSSKPIGPDDAIDALSSDF 239 Query 227 TCGSPTAAGKKTE 239 ||||||||||||| Query 227 TCGSPTAAGKKTE 239 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL-----#AKPIGP--------------DDAIDAL 222 | + + + ++||+|+ ||||+|| || # ||+ | | |||| Sbjct 595 VKEKAKAEHRDKLGERDDTIPPEYRHLLDDNGQ#DKPVKPPTKKSEDSKKPADDQDPIDAL 654 Query 223 SSDF-TCGSPTAAGKKT 238 | | +| | | + | Sbjct 655 SGDLDSCPSTTETSQNT 671 |
| Ovis aries | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL-------------#AKPIGPDDAIDALSSDF 226 |||||||||||||||||||||+|||||||| #+||+||+|||||||||| Sbjct 182 PEVSDPMSSTYIEELGKREVTLPPKYRELLNKEEGIAGPPPDS#SKPLGPNDAIDALSSDF 241 Query 227 TCGSPTAAGKKTE 239 || |||| |||| Sbjct 242 TCSSPTADAKKTE 254 Query 192 EELGKREVTIPPKYRELL#AK-----------------PIGPDDAIDALSSDFTCGSPTAA 234 |+||+|| |||| || #|| |+ | +|||| ||| | |++ Sbjct 471 EKLGEREETIPPDYRLEE#AKDKDGKPLPPKEVKEPLPPLSEDFLLDALSKDFTVPSDTSS 530 |
| Bos taurus | Query 180 PEVSDPMSSTYIEELGKREVTIPPKYRELL#A-------------KPIGPDDAIDALSSDF 226 ||||||||||||||||||| |+||||+|||# ||+||+|||||||||| Sbjct 182 PEVSDPMSSTYIEELGKRESTLPPKYKELL#NKEEGIAGPPPDSLKPLGPNDAIDALSSDF 241 Query 227 TCGSPTAAGKKTE 239 || |||| |||| Sbjct 242 TCSSPTADAKKTE 254 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL--------------------#AKPIGPDDAIDA 221 | + + + ++||+|+ ||||||+ || # || | | ||| Sbjct 599 VKEKAKAEHRDKLGERDDTIPPKYQHLLDDNKEGTPGKPKASEKPKAS#EKPAGAQDPIDA 658 Query 222 LSSDF-TCGSPT 232 || || +| | | Sbjct 659 LSGDFDSCPSTT 670 Query 192 EELGKREVTIPPKYRELL#AK-----------------PIGPDDAIDALSSDFTCGSPTAA 234 |+||++| |||| || #|| |+ | +|||| ||| | |++ Sbjct 471 EKLGEKEETIPPDYRLEE#AKDKDGKPLLPKEVKEPLPPLSEDVLLDALSKDFTVPSDTSS 530 |
[Site 4] IPPKYRELLA210-KPIGPDDAID
Ala210  Lys
Lys
 |
|||||||||
| P10 | P9 | P8 | P7 | P6 | P5 | P4 | P3 | P2 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| Ile201 | Pro202 | Pro203 | Lys204 | Tyr205 | Arg206 | Glu207 | Leu208 | Leu209 | Ala210 |
 |
|||||||||
| P1' | P2' | P3' | P4' | P5' | P6' | P7' | P8' | P9' | P10' |
| Lys211 | Pro212 | Ile213 | Gly214 | Pro215 | Asp216 | Asp217 | Ala218 | Ile219 | Asp220 |
 Sequence conservation (by blast)
Sequence conservation (by blast) Sequence conservation (by blast)
Sequence conservation (by blast)
| Reference peptide (cleaved bond±30 residues) |
|---|
| EVSDPMSSTYIEELGKREVTIPPKYRELLAKPIGPDDAIDALSSDFTCGSPTAAGKKTEK |
Summary
| # | organism | max score | hits | top seq |
|---|---|---|---|---|
| 1 | N/A | 123.00 | 9 | calpastatin |
| 2 | Homo sapiens | 116.00 | 9 | calpastatin |
| 3 | Mus musculus | 72.40 | 12 | calpastatin |
| 4 | Bos taurus | 48.90 | 8 | calpastatin |
| 5 | Rattus norvegicus | 47.40 | 7 | calpastatin |
| 6 | Xenopus laevis | 40.40 | 1 | putative calpastatin |
| 7 | Xenopus laevis | 38.10 | 2 | calpastatin |
| 8 | Oryctolagus cuniculus | 37.00 | 1 | calpastatin |
| 9 | Rattus sp. | 36.20 | 1 | calpastatin/CANP inhibitor |
| 10 | Pongo pygmaeus | 35.80 | 2 | hypothetical protein |
| 11 | Homo sapiens | 35.80 | 2 | calpastatin isoform i |
| 12 | Sus scrofa | 35.00 | 3 | type III calpastatin |
| 13 | Ovis aries | 34.70 | 2 | calpastatin |
| 14 | Bos taurus | 32.70 | 1 | calpastatin isoform III |
| 15 | Canis familiaris | 30.80 | 4 | PREDICTED: similar to calpastatin isoform a isofo |
| 16 | Rattus norvegicus | 30.40 | 1 | calpastatin isoform c |
Top-ranked sequences
| organism | matching |
|---|---|
| N/A | Query 181 EVSDPMSSTYIEELGKREVTIPPKYRELLA#KPIGPDDAIDALSSDFTCGSPTAAGKKTEK 240 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 181 EVSDPMSSTYIEELGKREVTIPPKYRELLA#KPIGPDDAIDALSSDFTCGSPTAAGKKTEK 240 |
| Homo sapiens | Query 181 EVSDPMSSTYIEELGKREVTIPPKYRELLA#K-------------PIGPDDAIDALSSDFT 227 ||||||||||||||||||||||||||||||#| |||||||||||||||| Sbjct 181 EVSDPMSSTYIEELGKREVTIPPKYRELLA#KKEGITGPPADSSKPIGPDDAIDALSSDFT 240 Query 228 CGSPTAAGKKTEK 240 ||||||||||||| Query 228 CGSPTAAGKKTEK 240 |
| Mus musculus | Query 182 VSDPMSSTYIEELGKREVTIPPKYRELLA-------------#KPIGPDDAIDALSSDFTC 228 | ||| |||+| || +| ||||+||+|| #||+| | ||||||||||| Sbjct 161 VLDPMDSTYLEALGIKEGTIPPEYRKLLEKNEGITQPLPDSP#KPMGTDQAIDALSSDFTC 220 Query 229 GSPTAAGKKTEK 240 ||| ||++|| Sbjct 221 SSPT--GKQSEK 230 |
| Bos taurus | Query 214 GPDDAIDALSSDFTCGSPTAAGKKTEK 240 ||+|||||||||||| |||| ||||| Sbjct 1 GPNDAIDALSSDFTCSSPTADAKKTEK 27 |
| Rattus norvegicus | Query 182 VSDPMSSTYIEELGKREVTIPPKYRELLA-------------#KPIGPDDAIDA 221 | ||| |||+| || +| ||||+||+|| #||+| | |||| Sbjct 123 VLDPMDSTYLEALGIKEETIPPEYRKLLEKNEAITGPLPDSP#KPMGIDHAIDA 175 |
| Xenopus laevis | Query 181 EVSDPMSSTYIEELGKREVTIPPKYRELL---------------A#KPIGPDDAIDALSSD 225 || + +| | ||||||| +||| || || #+|+ | || | | Sbjct 92 EVMETSTSEYREELGKREGSIPPAYRHLLDGKVDGKVAPPPPPEE#EPMTDDQLIDEFSMD 151 Query 226 FTC 228 |+| Sbjct 152 FSC 154 |
| Xenopus laevis | Query 181 EVSDPMSSTYIEELGKREVTIPPKYRELL-----------A#KPIGPD------DAIDALS 223 ||++ + |||+||||||+ ||||+|| || +# |+ |+ | +| | Sbjct 397 EVAETIKSTYLEELGKRDHTIPPEYRNLLDGKDHEKAVQPS#APVEPEPTMTDADLVDEFS 456 Query 224 SDF--TCGSPT 232 || +| ||| Sbjct 457 KDFELSC-SPT 466 Query 186 MSSTYIEELGKREVTIPPKYRELL-------A#KPIGP---------DDAIDALSSDF--- 226 ++| |+||||||+ |||| ||+|| |# | | || + |||| | Sbjct 242 VTSKYVEELGKRDHTIPPNYRKLLDGKGEKMA#PPTPPLVAEASMDDDDLVAALSSGFKSS 301 Query 227 -TC 228 || Sbjct 302 QTC 304 Query 181 EVSDPMSSTYIEELGKREVTIPPKYRELL---------------A#KPIGPDDAIDALSSD 225 ||++ ++ | ||||||+ +||| || || #+|+ | +| | | Sbjct 535 EVTETSTAVYREELGKRDDSIPPAYRHLLDGKEDGKVAPPPPPEE#EPLTDDQLLDEFSMD 594 Query 226 FTC 228 |+| Sbjct 595 FSC 597 Query 190 YIEELGKREVTIPPKYRELL--------------------A#KPIGPDDAIDALSSDF 226 +|+ || |+ ||||+||+|| #||+ | ||||||| | Sbjct 820 HIDRLGDRDDTIPPEYRKLLDGKDDKGQAAKPPVKEEEKPK#KPLSDDAAIDALSSGF 876 |
| Oryctolagus cuniculus | Query 181 EVSDPMSSTYIEELGKREVTIPPKYRELLA#K-------------PIGPDDAIDALSSDFT 227 |+||||||||||||||||||||||||||| #| |+|||||||||||||| Sbjct 181 EISDPMSSTYIEELGKREVTIPPKYRELLE#KKTGVAGPPPDSVTPLGPDDAIDALSSDFT 240 Query 228 CGSPTAAGK 236 | || |+|| Sbjct 241 CSSPVASGK 249 Query 190 YIEELGKREVTIPPKYRELL-------------------A#KPIGPDDAIDALSSDFTCGS 230 + ++||+|+ ||||+|| || #|| | | ||||| | Sbjct 607 HKDKLGERDDTIPPEYRHLLDQGEQDKPEKPPTKKSKEIK#KPAGDQDPIDALSGDLDSCP 666 Query 231 PTA 233 | | Sbjct 667 PAA 669 |
| Rattus sp. | Query 182 VSDPMSSTYIEELGKREVTIPPKYRELLA-------------#KPIGPDDAIDALSSDFTC 228 | ||| |||+| || +| ||||+||+|| #||+| | ||||||||||| Sbjct 110 VLDPMDSTYLEALGIKEGTIPPEYRKLLEKNEAITGPLPDSP#KPMGIDHAIDALSSDFTC 169 Query 229 GSPTAAGKKTEK 240 ||| ||+||| Sbjct 170 SSPT--GKQTEK 179 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL--------A#KPI-------GPD-DAIDALSSD 225 | + + + + |+||+|+ ||||+|| || #||+ | | | ||||| | Sbjct 490 VKEKIKAEHSEKLGERDDTIPPEYRHLLDNDGKDKPE#KPLDKEHREAGQDQDPIDALSED 549 Query 226 FTCGSPT 232 || Sbjct 550 LDSCPPT 556 |
| Pongo pygmaeus | Query 181 EVSDPMSSTYIEELGKREVTIPPKYRELLA#K-------------PIGPDDAIDALSSDFT 227 |||||||||||||||+||||||||||||||#| |+|||||||||||||| Sbjct 167 EVSDPMSSTYIEELGEREVTIPPKYRELLA#KNEGITGPPPDSSKPMGPDDAIDALSSDFT 226 Query 228 CGSPTAAGKKTEK 240 ||||||||||||| Query 228 CGSPTAAGKKTEK 240 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL-----A#KPIGP--------------DDAIDAL 222 | + + + ++||+|+ ||||+|| || #||+ | | |||| Sbjct 580 VKEKAKAEHRDKLGERDDTIPPEYRHLLDDNGQD#KPVKPPTKKSEDSKKPADDQDPIDAL 639 Query 223 SSDF-TCGSPTAAGKKTEK 240 | | +| | | + | | Sbjct 640 SGDLDSCPSTTETSQNTAK 658 |
| Homo sapiens | Query 181 EVSDPMSSTYIEELGKREVTIPPKYRELLA#K-------------PIGPDDAIDALSSDFT 227 ||||||||||||||||||||||||||||||#| |||||||||||||||| Sbjct 181 EVSDPMSSTYIEELGKREVTIPPKYRELLA#KKEGITGPPADSSKPIGPDDAIDALSSDFT 240 Query 228 CGSPTAAGKKTEK 240 ||||||||||||| Query 228 CGSPTAAGKKTEK 240 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL-----A#KPIGP--------------DDAIDAL 222 | + + + ++||+|+ ||||+|| || #||+ | | |||| Sbjct 595 VKEKAKAEHRDKLGERDDTIPPEYRHLLDDNGQD#KPVKPPTKKSEDSKKPADDQDPIDAL 654 Query 223 SSDF-TCGSPTAAGKKTEK 240 | | +| | | + | | Sbjct 655 SGDLDSCPSTTETSQNTAK 673 |
| Sus scrofa | Query 181 EVSDPMSSTYIEELGKREVTIPPKYRELL-------------A#KPIGPDDAIDALSSDFT 227 || |||||||||||||||||+|||||||| +#||+|||||||||| | | Sbjct 160 EVLDPMSSTYIEELGKREVTLPPKYRELLDKKEGIPVPPPDTS#KPLGPDDAIDALSLDLT 219 Query 228 CGSPTAAGKKTEK 240 | |||| |||||| Sbjct 220 CSSPTADGKKTEK 232 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL-------------------A#KPIGPDDAIDAL 222 | + + + ++||+|+ ||||+|| || #|| | |||| Sbjct 573 VKEKAEAEHRDKLGERDDTIPPEYRHLLDKDEEGKSTKPPTKKPEAPK#KPEAAQDPIDAL 632 Query 223 SSDF 226 | || Sbjct 633 SGDF 636 |
| Ovis aries | Query 222 LSSDFTCGSPTAAGKKTEK 240 ||||||| |||| ||||| Sbjct 1 LSSDFTCSSPTADAKKTEK 19 |
| Bos taurus | Query 181 EVSDPMSSTYIEELGKREVTIPPKYRELLA-------------#KPIGPDDAIDALSSDFT 227 |||||||||||||||||| |+||||+||| #||+||+||||||||||| Sbjct 183 EVSDPMSSTYIEELGKRESTLPPKYKELLNKEEGIAGPPPDSL#KPLGPNDAIDALSSDFT 242 Query 228 CGSPTAAGKKTEK 240 | |||| ||||| Sbjct 243 CSSPTADAKKTEK 255 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL--------------------A#KPIGPDDAIDA 221 | + + + ++||+|+ ||||||+ || #|| | | ||| Sbjct 599 VKEKAKAEHRDKLGERDDTIPPKYQHLLDDNKEGTPGKPKASEKPKASE#KPAGAQDPIDA 658 Query 222 LSSDF-TCGSPT 232 || || +| | | Sbjct 659 LSGDFDSCPSTT 670 Query 192 EELGKREVTIPPKYRELLA#K-----------------PIGPDDAIDALSSDFTCGSPTAA 234 |+||++| |||| || |#| |+ | +|||| ||| | |++ Sbjct 471 EKLGEKEETIPPDYRLEEA#KDKDGKPLLPKEVKEPLPPLSEDVLLDALSKDFTVPSDTSS 530 |
| Canis familiaris | Query 181 EVSDPMSSTYIEELGKREVTIPPKYRELLA#KPIGPDDAIDALSSDFTCGSPTAAGKKT 238 |+||||+|||||||||||||+|||||||||#||+||+|||||||||||| ||| ||||| Sbjct 226 EISDPMTSTYIEELGKREVTLPPKYRELLA#KPMGPNDAIDALSSDFTCSSPTDAGKKT 283 Query 190 YIEELGKREVTIPPKYRELL------------------A#KPIGPDDAIDALSSDF-TCGS 230 + ++||+|+ ||||+|| || #|| | ||||| || +| + Sbjct 638 HRDKLGERDDTIPPEYRHLLDNDEGKPGKPPAKKTKDSK#KPEDDKDPIDALSGDFDSCPA 697 Query 231 PTAAGKKT 238 || | + | Sbjct 698 PTEASENT 705 Query 192 EELGKREVTIPPKYR----------ELLA#K-------PIGPDDAIDALSSDFTCGSPTA 233 |+||++| |||| || || #| |+ | +|||| || | |+ Sbjct 502 EKLGEKEETIPPDYRLEEVKDKDGKPLLP#KVPKESLLPMSEDFLLDALSKDFAGSSNTS 560 |
| Rattus norvegicus | Query 182 VSDPMSSTYIEELGKREVTIPPKYRELLA-------------#KPIGPDDAIDALSSDFTC 228 | ||| |||+| || +| ||||+||+|| #||+| | ||||||||||| Sbjct 159 VLDPMDSTYLEALGIKEGTIPPEYRKLLEKNEAITGPLPDSP#KPMGIDHAIDALSSDFTC 218 Query 229 GSPTAAGKKTEK 240 ||| ||+||| Sbjct 219 SSPT--GKQTEK 228 Query 182 VSDPMSSTYIEELGKREVTIPPKYRELL----------------A#KPIGPDDAIDALSSD 225 | + + + + |+||+|+ ||||+|| || #|| | ||||| | Sbjct 539 VKEKIKAEHSEKLGERDDTIPPEYRHLLDNDGKDKPEKPLTKNTE#KPGQDQDPIDALSED 598 Query 226 FTCGSPT 232 || Sbjct 599 LDSCPPT 605 Query 192 EELGKREVTIPPKYR-ELL----A#KPIGPDDA------------IDALSSDFTC 228 |+||++| |||| || |++ #||+ | +| +|||| ||+ Sbjct 412 EKLGEKEETIPPDYRLEIVKDKDG#KPLLPKEAEEQLPPLSDDFLLDALSQDFSS 465 |
[Site 5] IDALSSDFTC228-GSPTAAGKKT
Cys228  Gly
Gly
 |
|||||||||
| P10 | P9 | P8 | P7 | P6 | P5 | P4 | P3 | P2 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| Ile219 | Asp220 | Ala221 | Leu222 | Ser223 | Ser224 | Asp225 | Phe226 | Thr227 | Cys228 |
 |
|||||||||
| P1' | P2' | P3' | P4' | P5' | P6' | P7' | P8' | P9' | P10' |
| Gly229 | Ser230 | Pro231 | Thr232 | Ala233 | Ala234 | Gly235 | Lys236 | Lys237 | Thr238 |
 Sequence conservation (by blast)
Sequence conservation (by blast) Sequence conservation (by blast)
Sequence conservation (by blast)
| Reference peptide (cleaved bond±30 residues) |
|---|
| VTIPPKYRELLAKPIGPDDAIDALSSDFTCGSPTAAGKKTEKEESTEVLKAQSAGTVRSA |
Summary
| # | organism | max score | hits | top seq |
|---|---|---|---|---|
| 1 | N/A | 122.00 | 7 | 1611327A calpastatin |
| 2 | Homo sapiens | 121.00 | 6 | AF327443_1 calpastatin |
| 3 | Homo sapiens | 112.00 | 2 | calpastatin isoform i |
| 4 | Pongo pygmaeus | 109.00 | 2 | hypothetical protein |
| 5 | Ovis aries | 89.40 | 3 | calpastatin |
| 6 | Sus scrofa | 87.40 | 3 | ICAL_PIG Calpastatin (Calpain inhibitor) gi |
| 7 | Mus musculus | 71.60 | 9 | calpastatin type III |
| 8 | Rattus norvegicus | 70.50 | 6 | calpastatin isoform a |
| 9 | Bos taurus | 70.10 | 8 | calpastatin |
| 10 | Rattus norvegicus | 70.10 | 1 | calpastatin isoform c |
| 11 | Rattus sp. | 69.70 | 1 | calpastatin/CANP inhibitor |
| 12 | Canis familiaris | 34.30 | 4 | PREDICTED: similar to calpastatin isoform a isofo |
| 13 | Bos taurus | 31.60 | 1 | calpastatin isoform III |
| 14 | Oryctolagus cuniculus | 31.20 | 1 | calpastatin |
Top-ranked sequences
| organism | matching |
|---|---|
| N/A | Query 199 VTIPPKYRELLAKPIGPDDAIDALSSDFTC#GSPTAAGKKTEKEESTEVLKAQSAGTVRSA 258 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 199 VTIPPKYRELLAKPIGPDDAIDALSSDFTC#GSPTAAGKKTEKEESTEVLKAQSAGTVRSA 258 |
| Homo sapiens | Query 199 VTIPPKYRELLAKPIGPDDAIDALSSDFTC#GSPTAAGKKTEKEESTEVLKAQSAGTVRSA 258 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 199 VTIPPKYRELLAKPIGPDDAIDALSSDFTC#GSPTAAGKKTEKEESTEVLKAQSAGTVRSA 258 |
| Homo sapiens | Query 199 VTIPPKYRELLAK-------------PIGPDDAIDALSSDFTC#GSPTAAGKKTEKEESTE 245 ||||||||||||| |||||||||||||||||#||||||||||||||||| Sbjct 199 VTIPPKYRELLAKKEGITGPPADSSKPIGPDDAIDALSSDFTC#GSPTAAGKKTEKEESTE 258 Query 246 VLKAQSAGTVRSA 258 ||||||||||||| Query 246 VLKAQSAGTVRSA 258 |
| Pongo pygmaeus | Query 199 VTIPPKYRELLAK-------------PIGPDDAIDALSSDFTC#GSPTAAGKKTEKEESTE 245 ||||||||||||| |+|||||||||||||||#||||||||||||||| | Sbjct 185 VTIPPKYRELLAKNEGITGPPPDSSKPMGPDDAIDALSSDFTC#GSPTAAGKKTEKEESIE 244 Query 246 VLKAQSAGTVRSA 258 ||||||||||||| Query 246 VLKAQSAGTVRSA 258 |
| Ovis aries | Query 199 VTIPPKYRELL-------------AKPIGPDDAIDALSSDFTC#GSPTAAGKKTEKEEST- 244 ||+|||||||| +||+||+||||||||||||# |||| ||||||+|| Sbjct 201 VTLPPKYRELLNKEEGIAGPPPDSSKPLGPNDAIDALSSDFTC#SSPTADAKKTEKEKSTE 260 Query 245 EVLKAQSAGTVRSA 258 | ||||||| +||| Sbjct 261 EALKAQSAGVIRSA 274 |
| Sus scrofa | Query 199 VTIPPKYRELL-------------AKPIGPDDAIDALSSDFTC#GSPTAAGKKTEKEEST- 244 ||+|||||||| +||+|||||||||| | ||# |||| |||||||+|| Sbjct 200 VTLPPKYRELLDKKEGIPVPPPDTSKPLGPDDAIDALSLDLTC#SSPTADGKKTEKEKSTG 259 Query 245 EVLKAQSAGTVRSA 258 ||||||| | ++|| Sbjct 260 EVLKAQSVGVIKSA 273 |
| Mus musculus | Query 200 TIPPKYRELLAK-------------PIGPDDAIDALSSDFTC#GSPTAAGKKTEKEEST-E 245 ||||+||+|| | |+| | |||||||||||# ||| ||++|||+|| | Sbjct 198 TIPPEYRKLLEKNEGITQPLPDSPKPMGTDQAIDALSSDFTC#SSPT--GKQSEKEKSTGE 255 Query 246 VLKAQSAGTVRSA 258 + |||||| ||+ Sbjct 256 IFKAQSAGVTRSS 268 |
| Rattus norvegicus | Query 200 TIPPKYRELLAK-------------PIGPDDAIDALSSDFTC#GSPTAAGKKTEKEEST-E 245 ||||+||+|| | |+| | |||||||||||# ||| ||+||||+|| | Sbjct 238 TIPPEYRKLLEKNEAITGPLPDSPKPMGIDHAIDALSSDFTC#SSPT--GKQTEKEKSTGE 295 Query 246 VLKAQSAGTVRSA 258 |||||| ||| Sbjct 296 SSKAQSAGVTRSA 308 |
| Bos taurus | Query 214 GPDDAIDALSSDFTC#GSPTAAGKKTEKEEST-EVLKAQSAGTVRSA 258 ||+||||||||||||# |||| ||||||+|| | ||||||| +||| Sbjct 1 GPNDAIDALSSDFTC#SSPTADAKKTEKEKSTEEALKAQSAGVIRSA 46 |
| Rattus norvegicus | Query 200 TIPPKYRELLAK-------------PIGPDDAIDALSSDFTC#GSPTAAGKKTEKEEST-E 245 ||||+||+|| | |+| | |||||||||||# ||| ||+||||+|| | Sbjct 177 TIPPEYRKLLEKNEAITGPLPDSPKPMGIDHAIDALSSDFTC#SSPT--GKQTEKEKSTGE 234 Query 246 VLKAQSAGTVRSA 258 |||||| ||| Sbjct 235 SSKAQSAGVTRSA 247 |
| Rattus sp. | Query 200 TIPPKYRELLAK-------------PIGPDDAIDALSSDFTC#GSPTAAGKKTEKEEST-E 245 ||||+||+|| | |+| | |||||||||||# ||| ||+||||+|| | Sbjct 128 TIPPEYRKLLEKNEAITGPLPDSPKPMGIDHAIDALSSDFTC#SSPT--GKQTEKEKSTGE 185 Query 246 VLKAQSAGTVRSA 258 |||||| ||| Sbjct 186 SSKAQSAGVTRSA 198 |
| Canis familiaris | Query 199 VTIPPKYRELLAKPIGPDDAIDALSSDFTC#GSPTAAGKKTEKEEST--EVLKAQSAGTVR 256 ||+|||||||||||+||+||||||||||||# ||| |||||++|+|| |+|||| | + Sbjct 244 VTLPPKYRELLAKPMGPNDAIDALSSDFTC#SSPTDAGKKTKEEKSTGEEILKAQPARVTK 303 Query 257 SA 258 || Query 257 SA 258 Query 200 TIPPKYRELL------------------AKPIGPDDAIDALSSDF-TC#GSPTAAGKKT 238 ||||+|| || || | ||||| || +|# +|| | + | Sbjct 648 TIPPEYRHLLDNDEGKPGKPPAKKTKDSKKPEDDKDPIDALSGDFDSC#PAPTEASENT 705 |
| Bos taurus | Query 200 TIPPKYRELLAK-------------PIGPDDAIDALSSDFTC#GSPTAAGKKTEKEEST-E 245 |+||||+||| | |+||+||||||||||||# |||| ||||||+|| | Sbjct 202 TLPPKYKELLNKEEGIAGPPPDSLKPLGPNDAIDALSSDFTC#SSPTADAKKTEKEKSTEE 261 Query 246 VLKAQSAGTVRSA 258 ||||||| +||| Sbjct 262 ALKAQSAGVIRSA 274 Query 200 TIPPKYRELL--------------------AKPIGPDDAIDALSSDF-TC#GSPTAAGKKT 238 ||||||+ || || | | ||||| || +|# | | | Sbjct 617 TIPPKYQHLLDDNKEGTPGKPKASEKPKASEKPAGAQDPIDALSGDFDSC#PSTTETSTDT 676 Query 239 EKE 241 |+ Sbjct 677 PKD 679 |
| Oryctolagus cuniculus | Query 199 VTIPPKYRELLAK-------------PIGPDDAIDALSSDFTC#GSPTAAGKKTEKEEST- 244 ||||||||||| | |+|||||||||||||||# || |+||+ || + Sbjct 199 VTIPPKYRELLEKKTGVAGPPPDSVTPLGPDDAIDALSSDFTC#SSPVASGKEAGKEAAKS 258 Query 245 --EVLKAQSAGTVRSA 258 |||+|+|| +|+| Sbjct 259 AGEVLEAESAKVMRAA 274 Query 200 TIPPKYRELL-------------------AKPIGPDDAIDALSSDFTC#GSPTA-AGKKTE 239 ||||+|| || || | | ||||| | # | | + || Sbjct 617 TIPPEYRHLLDQGEQDKPEKPPTKKSKEIKKPAGDQDPIDALSGDLDS#CPPAAETSQATE 676 Query 240 KEES 243 |++| Sbjct 677 KDKS 680 |
[Site 6] TEVLKAQSAG253-TVRSAAPPQE
Gly253  Thr
Thr
 |
|||||||||
| P10 | P9 | P8 | P7 | P6 | P5 | P4 | P3 | P2 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| Thr244 | Glu245 | Val246 | Leu247 | Lys248 | Ala249 | Gln250 | Ser251 | Ala252 | Gly253 |
 |
|||||||||
| P1' | P2' | P3' | P4' | P5' | P6' | P7' | P8' | P9' | P10' |
| Thr254 | Val255 | Arg256 | Ser257 | Ala258 | Ala259 | Pro260 | Pro261 | Gln262 | Glu263 |
 Sequence conservation (by blast)
Sequence conservation (by blast) Sequence conservation (by blast)
Sequence conservation (by blast)
| Reference peptide (cleaved bond±30 residues) |
|---|
| SDFTCGSPTAAGKKTEKEESTEVLKAQSAGTVRSAAPPQEKKRKVEKDTMSDQALEALSA |
Summary
| # | organism | max score | hits | top seq |
|---|---|---|---|---|
| 1 | Homo sapiens | 119.00 | 6 | calpastatin |
| 2 | N/A | 118.00 | 7 | 1611327A calpastatin |
| 3 | Homo sapiens | 118.00 | 2 | calpastatin isoform i |
| 4 | Pongo pygmaeus | 116.00 | 2 | hypothetical protein |
| 5 | Bos taurus | 93.20 | 5 | calpastatin isoform II |
| 6 | Bos taurus | 93.20 | 1 | calpastatin isoform III |
| 7 | Ovis aries | 92.00 | 3 | calpastatin |
| 8 | Sus scrofa | 89.00 | 3 | type III calpastatin |
| 9 | Canis familiaris | 84.30 | 1 | PREDICTED: similar to calpastatin isoform a isofo |
| 10 | Oryctolagus cuniculus | 79.70 | 1 | calpastatin |
| 11 | Rattus norvegicus | 79.30 | 6 | calpastatin isoform a |
| 12 | Rattus norvegicus | 79.30 | 1 | calpastatin isoform c |
| 13 | Rattus sp. | 79.00 | 1 | calpastatin/CANP inhibitor |
| 14 | Mus musculus | 62.00 | 9 | calpastatin |
| 15 | Brassica napus | 31.20 | 1 | BnMAP4K alpha1 |
Top-ranked sequences
| organism | matching |
|---|---|
| Homo sapiens | Query 224 SDFTCGSPTAAGKKTEKEESTEVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALSA 283 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 224 SDFTCGSPTAAGKKTEKEESTEVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALSA 283 |
| N/A | Query 224 SDFTCGSPTAAGKKTEKEESTEVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALSA 283 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 224 SDFTCGSPTAAGKKTEKEESTEVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALSA 283 |
| Homo sapiens | Query 224 SDFTCGSPTAAGKKTEKEESTEVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALSA 283 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 224 SDFTCGSPTAAGKKTEKEESTEVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALSA 283 |
| Pongo pygmaeus | Query 224 SDFTCGSPTAAGKKTEKEESTEVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALSA 283 |||||||||||||||||||| |||||||||#|||||||||||||||||||||||||||||| Sbjct 223 SDFTCGSPTAAGKKTEKEESIEVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALSA 282 |
| Bos taurus | Query 224 SDFTCGSPTAAGKKTEKEESTE-VLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALS 282 ||||| |||| ||||||+||| |||||||# +|||||||||||||||| |++ |||||| Sbjct 307 SDFTCSSPTADAKKTEKEKSTEEALKAQSAG#VIRSAAPPQEKKRKVEKDAMTEHALEALS 366 Query 283 A 283 | Query 283 A 283 |
| Bos taurus | Query 224 SDFTCGSPTAAGKKTEKEESTE-VLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALS 282 ||||| |||| ||||||+||| |||||||# +|||||||||||||||| |++ |||||| Sbjct 239 SDFTCSSPTADAKKTEKEKSTEEALKAQSAG#VIRSAAPPQEKKRKVEKDAMTEHALEALS 298 Query 283 A 283 | Query 283 A 283 |
| Ovis aries | Query 224 SDFTCGSPTAAGKKTEKEESTE-VLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALS 282 ||||| |||| ||||||+||| |||||||# +||||||+||+||||+|||++||||||| Sbjct 239 SDFTCSSPTADAKKTEKEKSTEEALKAQSAG#VIRSAAPPKEKRRKVEEDTMTEQALEALS 298 Query 283 A 283 | Query 283 A 283 |
| Sus scrofa | Query 225 DFTCGSPTAAGKKTEKEEST-EVLKAQSAG#TVRSAA-PPQEKKRKVEKDTMSDQALEALS 282 | || |||| |||||||+|| ||||||| |# ++||| || ||||+||+|||||||||||| Sbjct 217 DLTCSSPTADGKKTEKEKSTGEVLKAQSVG#VIKSAAAPPHEKKRRVEEDTMSDQALEALS 276 Query 283 A 283 | Query 283 A 283 |
| Canis familiaris | Query 224 SDFTCGSPTAAGKKTEKEEST--EVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEAL 281 ||||| ||| |||||++|+|| |+|||| | # +||| |||||||||+| ||||||||| Sbjct 269 SDFTCSSPTDAGKKTKEEKSTGEEILKAQPAR#VTKSAASPQEKKRKVEEDAMSDQALEAL 328 Query 282 SA 283 || Query 282 SA 283 |
| Oryctolagus cuniculus | Query 224 SDFTCGSPTAAGKKTEKE---ESTEVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEA 280 ||||| || |+||+ || + |||+|+|| # +|+||||||||||||+| |||||||| Sbjct 237 SDFTCSSPVASGKEAGKEAAKSAGEVLEAESAK#VMRAAAPPQEKKRKVEEDAMSDQALEA 296 Query 281 LSA 283 ||| Query 281 LSA 283 |
| Rattus norvegicus | Query 224 SDFTCGSPTAAGKKTEKEEST-EVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALS 282 ||||| ||| ||+||||+|| | ||||||# ||| ||||||||||++ |+||||+||| Sbjct 275 SDFTCSSPT--GKQTEKEKSTGESSKAQSAG#VTRSAVPPQEKKRKVEEEVMNDQALQALS 332 |
| Rattus norvegicus | Query 224 SDFTCGSPTAAGKKTEKEEST-EVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALS 282 ||||| ||| ||+||||+|| | ||||||# ||| ||||||||||++ |+||||+||| Sbjct 214 SDFTCSSPT--GKQTEKEKSTGESSKAQSAG#VTRSAVPPQEKKRKVEEEVMNDQALQALS 271 |
| Rattus sp. | Query 224 SDFTCGSPTAAGKKTEKEEST-EVLKAQSAG#TVRSAAPPQEKKRKVEKDTMSDQALEALS 282 ||||| ||| ||+||||+|| | ||||||# ||| ||||||||||++ |+||||+||| Sbjct 165 SDFTCSSPT--GKQTEKEKSTGESSKAQSAG#VTRSAVPPQEKKRKVEEEVMNDQALQALS 222 |
| Mus musculus | Query 224 SDFTCGSPTAAGKKTEKEEST-EVLKAQSAG#TVRSAAPPQEKKRKVE 269 ||||| ||| ||++|||+|| |+ ||||||# ||+ ||+||||||| Sbjct 216 SDFTCSSPT--GKQSEKEKSTGEIFKAQSAG#VTRSSVPPKEKKRKVE 260 |
| Brassica napus | Query 249 AQSAG#TVRSAAPPQEKKRKVEKDTMSDQALE 279 +||||#|||+ ||| ++|+ | |||+ + Sbjct 342 SQSAG#TVRALKPPQSRERRQE--VTSDQSFQ 370 |
[Site 7] LKAQSAGTVR256-SAAPPQEKKR
Arg256  Ser
Ser
 |
|||||||||
| P10 | P9 | P8 | P7 | P6 | P5 | P4 | P3 | P2 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| Leu247 | Lys248 | Ala249 | Gln250 | Ser251 | Ala252 | Gly253 | Thr254 | Val255 | Arg256 |
 |
|||||||||
| P1' | P2' | P3' | P4' | P5' | P6' | P7' | P8' | P9' | P10' |
| Ser257 | Ala258 | Ala259 | Pro260 | Pro261 | Gln262 | Glu263 | Lys264 | Lys265 | Arg266 |
 Sequence conservation (by blast)
Sequence conservation (by blast) Sequence conservation (by blast)
Sequence conservation (by blast)
| Reference peptide (cleaved bond±30 residues) |
|---|
| TCGSPTAAGKKTEKEESTEVLKAQSAGTVRSAAPPQEKKRKVEKDTMSDQALEALSASLG |
Summary
| # | organism | max score | hits | top seq |
|---|---|---|---|---|
| 1 | N/A | 117.00 | 7 | 1611327A calpastatin |
| 2 | Homo sapiens | 117.00 | 6 | calpastatin isoform b |
| 3 | Homo sapiens | 117.00 | 2 | calpastatin isoform i |
| 4 | Pongo pygmaeus | 115.00 | 2 | hypothetical protein |
| 5 | Bos taurus | 92.00 | 5 | calpastatin type II |
| 6 | Bos taurus | 92.00 | 1 | calpastatin isoform III |
| 7 | Sus scrofa | 91.70 | 3 | type III calpastatin |
| 8 | Ovis aries | 90.90 | 3 | calpastatin |
| 9 | Canis familiaris | 83.20 | 1 | PREDICTED: similar to calpastatin isoform a isofo |
| 10 | Oryctolagus cuniculus | 78.20 | 1 | calpastatin |
| 11 | Rattus norvegicus | 77.80 | 6 | calpastatin isoform a |
| 12 | Rattus sp. | 77.40 | 1 | calpastatin/CANP inhibitor |
| 13 | Rattus norvegicus | 77.40 | 1 | calpastatin isoform c |
| 14 | Mus musculus | 58.50 | 9 | unnamed protein product |
| 15 | Brassica napus | 31.60 | 1 | BnMAP4K alpha1 |
Top-ranked sequences
| organism | matching |
|---|---|
| N/A | Query 227 TCGSPTAAGKKTEKEESTEVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASLG 286 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 227 TCGSPTAAGKKTEKEESTEVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASLG 286 |
| Homo sapiens | Query 227 TCGSPTAAGKKTEKEESTEVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASLG 286 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 227 TCGSPTAAGKKTEKEESTEVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASLG 286 |
| Homo sapiens | Query 227 TCGSPTAAGKKTEKEESTEVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASLG 286 ||||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 227 TCGSPTAAGKKTEKEESTEVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASLG 286 |
| Pongo pygmaeus | Query 227 TCGSPTAAGKKTEKEESTEVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASLG 286 ||||||||||||||||| ||||||||||||#|||||||||||||||||||||||||||||| Sbjct 226 TCGSPTAAGKKTEKEESIEVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASLG 285 |
| Bos taurus | Query 227 TCGSPTAAGKKTEKEESTE-VLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASL 285 || |||| ||||||+||| ||||||| +|#||||||||||||||| |++ ||||||||| Sbjct 310 TCSSPTADAKKTEKEKSTEEALKAQSAGVIR#SAAPPQEKKRKVEKDAMTEHALEALSASL 369 Query 286 G 286 | Query 286 G 286 |
| Bos taurus | Query 227 TCGSPTAAGKKTEKEESTE-VLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASL 285 || |||| ||||||+||| ||||||| +|#||||||||||||||| |++ ||||||||| Sbjct 242 TCSSPTADAKKTEKEKSTEEALKAQSAGVIR#SAAPPQEKKRKVEKDAMTEHALEALSASL 301 Query 286 G 286 | Query 286 G 286 |
| Sus scrofa | Query 227 TCGSPTAAGKKTEKEEST-EVLKAQSAGTVR#SAA-PPQEKKRKVEKDTMSDQALEALSAS 284 || |||| |||||||+|| ||||||| | ++#||| || ||||+||+|||||||||||||| Sbjct 219 TCSSPTADGKKTEKEKSTGEVLKAQSVGVIK#SAAAPPHEKKRRVEEDTMSDQALEALSAS 278 Query 285 LG 286 || Query 285 LG 286 |
| Ovis aries | Query 227 TCGSPTAAGKKTEKEESTE-VLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASL 285 || |||| ||||||+||| ||||||| +|#|||||+||+||||+|||++|||||||||| Sbjct 242 TCSSPTADAKKTEKEKSTEEALKAQSAGVIR#SAAPPKEKRRKVEEDTMTEQALEALSASL 301 Query 286 G 286 | Query 286 G 286 |
| Canis familiaris | Query 227 TCGSPTAAGKKTEKEEST--EVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSAS 284 || ||| |||||++|+|| |+|||| | +#||| |||||||||+| |||||||||||| Sbjct 272 TCSSPTDAGKKTKEEKSTGEEILKAQPARVTK#SAASPQEKKRKVEEDAMSDQALEALSAS 331 Query 285 LG 286 || Query 285 LG 286 |
| Oryctolagus cuniculus | Query 227 TCGSPTAAGKKTEKE---ESTEVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSA 283 || || |+||+ || + |||+|+|| +|#+||||||||||||+| ||||||||||| Sbjct 240 TCSSPVASGKEAGKEAAKSAGEVLEAESAKVMR#AAAPPQEKKRKVEEDAMSDQALEALSA 299 Query 284 SLG 286 ||| Query 284 SLG 286 |
| Rattus norvegicus | Query 227 TCGSPTAAGKKTEKEEST-EVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASL 285 || ||| ||+||||+|| | |||||| |#|| ||||||||||++ |+||||+||| || Sbjct 278 TCSSPT--GKQTEKEKSTGESSKAQSAGVTR#SAVPPQEKKRKVEEEVMNDQALQALSDSL 335 Query 286 G 286 | Query 286 G 286 |
| Rattus sp. | Query 227 TCGSPTAAGKKTEKEEST-EVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASL 285 || ||| ||+||||+|| | |||||| |#|| ||||||||||++ |+||||+||| || Sbjct 168 TCSSPT--GKQTEKEKSTGESSKAQSAGVTR#SAVPPQEKKRKVEEEVMNDQALQALSDSL 225 Query 286 G 286 | Query 286 G 286 |
| Rattus norvegicus | Query 227 TCGSPTAAGKKTEKEEST-EVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASL 285 || ||| ||+||||+|| | |||||| |#|| ||||||||||++ |+||||+||| || Sbjct 217 TCSSPT--GKQTEKEKSTGESSKAQSAGVTR#SAVPPQEKKRKVEEEVMNDQALQALSDSL 274 Query 286 G 286 | Query 286 G 286 |
| Mus musculus | Query 227 TCGSPTAAGKKTEKEEST-EVLKAQSAGTVR#SAAPPQEKKRKVEKDTMSDQALEALSASL 285 || ||| ||++|||+|| |+ |||||| |#|+ ||+|||||||++ ++||||+||| || Sbjct 219 TCSSPT--GKQSEKEKSTGEIFKAQSAGVTR#SSVPPKEKKRKVEEEVINDQALQALSDSL 276 Query 286 G 286 | Query 286 G 286 |
| Brassica napus | Query 249 AQSAGTVR#SAAPPQEKKRKVEKDTMSDQALE 279 +|||||||#+ ||| ++|+ | |||+ + Sbjct 342 SQSAGTVR#ALKPPQSRERRQE--VTSDQSFQ 370 |
* References
[PubMed ID: 24462690] Sun W, Feng R, Hu H, Guo F, Gao Q, Shao D, Yin D, Wang H, Sun X, Zhao M, Minobe E, Sun Y, Jiao G, Kameyama M, Hao L, The Ca(2+)-dependent interaction of calpastatin domain L with the C-terminal tail of the Cav1.2 channel. FEBS Lett. 2014 Mar 3;588(5):665-71. doi: 10.1016/j.febslet.2014.01.019. Epub
[PubMed ID: 23951044] ... Zhang L, Ding H, Wang DH, Zhang YL, Baskys A, Chan P, Zhong Y, Cai YN, Calpastatin gene (CAST) is not associated with late onset sporadic Parkinson's disease in the Han Chinese population. PLoS One. 2013 Aug 9;8(8):e70935. doi: 10.1371/journal.pone.0070935. eCollection
[PubMed ID: 15820218] ... Raynaud P, Jayat-Vignoles C, Laforet MP, Leveziel H, Amarger V, Four promoters direct expression of the calpastatin gene. Arch Biochem Biophys. 2005 May 1;437(1):69-77.
[PubMed ID: 11978196] ... Zhu H, Zhou ZM, Li JM, Zhu H, Cheng LJ, Shan YX, Yin LL, Sha JH, Cloning and characterization of a novel isoform of calpastatin in human adult testis. Acta Pharmacol Sin. 2002 May;23(5):450-4.
[PubMed ID: 10859257] ... Li S, Liang ZG, Wang GY, Yavetz B, Kim ED, Goldberg E, Molecular cloning and characterization of functional domains of a human testis-specific isoform of calpastatin. Biol Reprod. 2000 Jul;63(1):172-8.
[PubMed ID: 1569094] ... Lee WJ, Ma H, Takano E, Yang HQ, Hatanaka M, Maki M, Molecular diversity in amino-terminal domains of human calpastatin by exon skipping. J Biol Chem. 1992 Apr 25;267(12):8437-42.
[PubMed ID: 1995645] ... Adachi Y, Ishida-Takahashi A, Takahashi C, Takano E, Murachi T, Hatanaka M, Phosphorylation and subcellular distribution of calpastatin in human hematopoietic system cells. J Biol Chem. 1991 Feb 25;266(6):3968-72.
[PubMed ID: 2407243] ... Uemori T, Shimojo T, Asada K, Asano T, Kimizuka F, Kato I, Maki M, Hatanaka M, Murachi T, Hanzawa H, et al., Characterization of a functional domain of human calpastatin. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1485-93.
[PubMed ID: 2265559] ... Inazawa J, Nakagawa H, Misawa S, Abe T, Minoshima S, Fukuyama R, Maki M, Murachi T, Hatanaka M, Shimizu N, Assignment of the human calpastatin gene (CAST) to chromosome 5 at region q14----q22. Cytogenet Cell Genet. 1990;54(3-4):156-8.
[PubMed ID: 2577276] ... Asada K, Ishino Y, Shimada M, Shimojo T, Endo M, Kimizuka F, Kato I, Maki M, Hatanaka M, Murachi T, cDNA cloning of human calpastatin: sequence homology among human, pig, and rabbit calpastatins. J Enzyme Inhib. 1989;3(1):49-56.