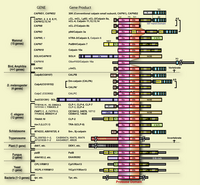
Calpain (EC 3.4.22.17, Clan CA, family C02) is an intracellular Ca2+-dependent cysteine protease, which is ubiquitously distributed, showing limited proteolytic activity at neutral pH. Calpain proteolytically processes, rather than digests, substrates to transform and modulate their structures and activities. Thus, calpain is placed as a representative intracellular “modulator protease” that governs various cellular functions such as signal transductions and cell morphogenesis. Calpain belongs to the papain superfamily, and constitutes one of its three distinct kingdoms, i.e., calpain-, papain-, and bleomycin-hydrolase-sub-superfamilies. Human has 15 genes that encode a calpain-like protease domain, and they generate diverse kinds of calpain homologues with combinations of several functional domains such as Ca2+-binding domain (C2-domain-type and EF-hand-type), Zn-finger domain, etc. Furthermore, calpain homologues are increasingly being found in other organisms including insect, nematode, trypanosome, plant, fungus, yeast, and even some bacteria, thus constituting a superfamily of versatile functions (see Fig. 1). Physiological functions of calpain are so important for the cells that their defects cause variety of deficiencies in many different organisms; muscular dystrophies, diabetes, and tumorigenesis in human, embryonic lethality in mouse, neurogenesis deficiency in fly, incomplete sex determination in nematodeys, defect of aleurone cell development in maize, and alkaline/osmo-shock susceptibility in yeast.
Calpain was described as early as in 1964 (Guroff 1964; Meyer et al. 1964). After several “re-identifications” (Dayton et al. 1976; Drummond & Duncan 1968; Huston & Krebs 1968), calpain, which was called CANP (calcium activated neutral protease) at that time, was finally purified into homogeneity in 1978 (Ishiura et al. 1978). Both names, ''calpain'' and ''CANP'', were unified as calpain in 1991 (Suzuki 1991). In 1984, cDNA for the catalytic subunit of calpain was cloned for the first time, revealing that calpain is a chimeric molecule of a cysteine protease and a calmodulin-like Ca2+-binding module (Ohno et al. 1984) (see Figs. 1 and 2). For the two decades from the time of this first cloning, more than hundreds of calpain-related molecules, including its endogenous specific inhibitor protein, calpastatin, have been identified by the cDNA cloning and genome/EST projects (see reviews such as (Goll et al. 2003; Ono et al. 1999; Sorimachi et al. 1997; Sorimachi & Suzuki 2001; Suzuki et al. 2004).
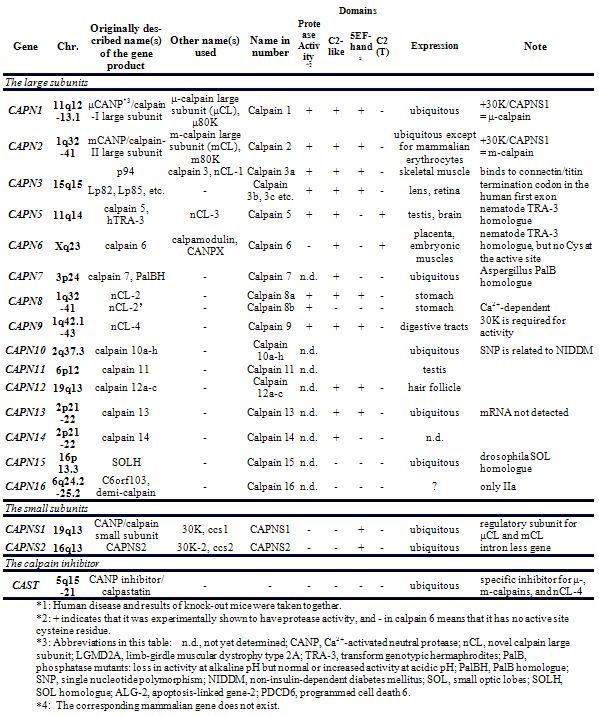
In mammals, two major ubiquitous calpains,μ- and m-calpains, exist (Fig. 2). Most of studies on calpain are focused on μ- and m-calpains, thus called “conventional” calpains. As the names suggest, μ-calpain and m-calpain are activated by μM and mM level of Ca2+ concentrations in vitro, respectively. They are a hetero-dimer of common calpain small regulatory subunit (30K, ca. 28 kDa) and distinct μ- and m-calpain large catalytic subunit (μCL and mCL, respectively, ca. 80 kDa), which are ca. 60 % identical in their amino acid sequences. Fifteen human calpain homologues are now called by numbers as in the case of caspases as shown in Table 1 and Fig. 3. In consequence, μCL is now called “calpain 1”, i.e., μ-calpain is a hetero-dimer of 30K and calpain 1. To avoid unnecessary confusions, however, the original name with the gene product name is adopted in this section, e.g., μCL/CAPN1, mCL/CAPN2, p94/CAPN3, 30K/CAPNS1, hTRA-3/calpain 5 etc.
Table. 1. Human calpain related genes
2. Structure and functions of conventional calpains
In general, potential substrates for calpains are very limited and specific, and most of oligo-peptides are not good substrates for calpains. Fortunately, casein is a very good in vitro substrate for calpains, and used to assay calpain activity, although casein should never be an in vivo substrate. The rules governing substrate specificity remain unclear, and calpain is considered to recognize a large scope of 3D structure of substrate rather than a primary sequence, although some preference in recognizing amino acid sequences have been reported (Brown & Crawford 1993; Stabach et al. 1997; Tompa et al. 2004). Protein kinases, phosphatases, phospholipases, cytoskeletal proteins, membrane proteins, cytokines, transcription factors, lens proteins, calmodulin-binding proteins, etc. are suggested to be in vivo substrates. There is no report suggesting the different substrate specificity between μ- and m-calpains.
Calpain has very specific proteinacious inhibitor named calpastatin in vivo (Goll et al. 2003). Calpastatin has four repeats of an inhibitor unit, each of which inhibits calpain. Both μ- and m-calpains have similar susceptibility to calpastatin.
μ- and m-calpains are ubiquitously expressed in mammalian and avian cells. Thus, the function is considered to be fundamental and essential. Many functions such as regulation of signal transduction system, cell motility, and apoptosis have been suggested so far although not completely clarified. Knock-out mice of Capn1 showed almost no phenotype, whereas knock-out of Capn2 or CAPNS1 caused embryonic lethality, indicating indispensable roles of conventional calpains and, at the same time, ability of m-calpain to complement functions of μ-calpain (Arthur et al. 2000; Azam et al. 2001; Dutt et al. 2006; Zimmerman et al. 2000).
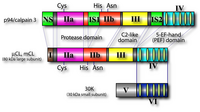
As shown in Fig. 1, the catalytic and regulatory subunits of conventional calpains can be divided into 4 and 2 domains, respectively. The N-terminus of domain I of the large subunit is autolyzed upon activation by Ca2+, to have lower requirement of Ca2+, and the autolysis results in dissociation of the both subunits. Therefore, autolysis is involved in regulation of calpain activity and specificity.
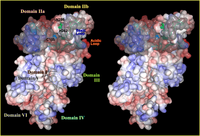
Three-dimensional structural studies revealed that the protease domain in the absence of Ca2+ is divided into two sub-domains, domains IIa and IIb, which are folded into one domain upon Ca2+ binding (Fig. 4). This domain is most conserved among calpain family members, suggesting indispensable functions of this domain (Fig. 1). Surprisingly, the protease domain of μ- and m-calpains without other domains showed Ca2+-dependent protease activity. This is supported by the 3D structural studies of the protease domain in the presence of Ca2+ that showed Ca2+ bound to domains IIa and IIb. Thus, Ca2+-dependency of calpains is controlled as a whole molecule, because all of domains IIa, IIb, III, IV, and VI bind at least one Ca2+ with different affinities.
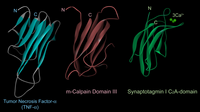
The 3D structure of domain III consists of 8 anti-parallel β-strands (β-sandwich structure), a structure very similar to TNF-α and C2-domains found in several Ca2+-regulated proteins such as PKCs and synaptotagmins (Fig. 5). Although the primary structure of domain III is highly conserved in calpain homologues, it has no similarity to any other proteins including TNF-α and C2-domains. This domain actually binds Ca2+, and may play an important role on Ca2+-dependent membrane translocation of calpains.
Domain IV is very similar to domain VI of the small subunit, and each contains 5 EF-hand motifs. Thus, these domains are called 5-EF-hand, or penta EF-hand (PEF) domain. in vitro experiments together with 3D structural studies showed that only the 1st, 2nd, and 3rd EF-hands bind Ca2+. The 5th EF-hand motif is rather involved in dimer formation of both subunits.
Domain V of the calpain regulatory subunit contains clusters of Gly making its nature hydrophobic. This domain is considered to interact with membrane and/or membrane proteins by hydrophobic interaction. Most of this domain is cut off by autolysis, indicating no involvement of this region in protease activity. In human, the other gene (CAPNS2) exists that encodes a regulatory subunit homologue, whose physiological roles are unclear.
3. Calpain superfamily and its members
(1) Classifications
As for calpain homologues other than conventional calpains, domains other than the protease domain are not necessarily conserved among homologues (Fig. 1). Amino acid sequence identities of the protease domains vary depending on molecules from less than 30 % to more than 75 %. Several kinds of domains putatively originated from independent genes exist in both N- and C-terminal parts of the protease domain. These include C2 and C2-like domains, 5-EF-hand domain, and trans-membrane domain as well as conserved domains with unknown functions (SOH, etc). These features together with gene organizations of mammalian calpain genes strongly suggest that calpain molecules are generated by combining the gene for the ancestral calpain-type cysteine protease and some of the genes for other functions (Hata et al. 2001).
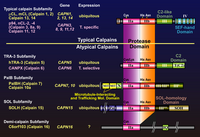
According to the similarity, these calpain homologues can be divided into two categories. The first group consists of molecules having domains II, III, and IV, i.e., the protease domain, the C2-like and 5-EF-hand Ca2+-binding domains. In other words, these group members have a “typical” structure highly similar to conventional calpain catalytic subunits. Beside mammalian μCL/CAPN1 and mCL/CAPN2, typical calpain includes p94/CAPN3, nCL-2/CAPN8, nCL-4/CAPN9, CAPN11, CAPN12, CAPN13, and CAPN14 (Fig. 3). Chicken as well as Xenopus laevis are reported to have μ/mCL in addition (Sorimachi et al. 1995). In invertebrates, only five have been identified as typical calpain so far. Three are found in Drosophila melanogaster as Dm-calpain (CALPA), CALPB, and CG-3692. Schistosome mansoni and S. japonicum also has at least one typical calpain (Sm-, and Sj-calpain). No typical calpain homologues have been found in Caenorhabtisis elegans, plants, fungi, trypanosomes, and Saccharomyces cerevisiae (Fig. 1).
The second group contains various molecules that have the protease domain but do not have domain III or IV. Instead, some possess extra domain(s) distinct from any domain I to VI. Thus, these molecules are “atypical” calpain homologues. These atypical calpains are thought to have somewhat different functions compared with those of typical calpains. Atypical calpain includes TRA-3 subfamily, SOL subfamily, PalB subfamily, alternative splicing products of Capn8 (nCL-2’) and CalpA, and others (Fig. 6).
In addition to the structural features, independent classification is possible according to their localization of the expression. In mammals, μCL/CAPN1, mCL/CAPN2, PalBH/CAPN7, CAPN10, and CAPN13 are ubiquitously expressed, whereas p94/CAPN3, nCL-2/CAPN8, nCL-4/CAPN9, hTRA-3/CAPN5, CAPN6, 10, 11, and 12 are predominantly expressed in restricted organs (Table 1).
(2). Structure and functions of calpain superfamily members
- Mammalian typical calpains other than conventional calpains
- Skeletal muscle-specific calpain, p94/CAPN3
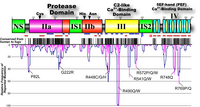
p94/CANP3 (often called “calpain 3” lately), the first tissue-specific calpain found in 1989 (Sorimachi et al. 1989), is ca. 60 % identical to the large subunits of μ- and m-calpains, and have conserved domain structure (Fig. 1). However, p94/CAPN3 contains three specific regions, NS, IS1, and IS2.
mRNA for p94/CAPN3 is expressed predominantly in skeletal muscle, and the amount is about 10 times larger than those for conventional calpain. p94/CAPN3 turned out to have several unique properties. First of all, p94/CAPN3 protein undergoes extremely rapid autolysis (half life in vitro is less than 10 minutes), and this autolysis is prohibited by deletion of either of p94-specific region, either IS1 or IS2 (Sorimachi et al. 1993b). Specific inhibitors to μ- and m-calpains such as calpastatin, E-64, and leupeptin have no effect on autolysis. p94/CAPN3 possesses nuclear localization signal-like sequence in IS2, and localizes in nucleus as well as cytosol. p94/CAPN3 binds to gigantic muscle protein, connectin/titin, specifically through IS2. Protease activity of p94/CAPN3 is regulated in vivo by binding to connectin/titin (Ono et al. 2006).
Some alternative splicing products (Lp82 etc.) of CAPN3 are specifically expressed in lenses. Lp82 showed Ca2+-dependent protease activity against βA3 and αB crystallins. Activity is inhibited by E-64, but not by calpastatin. Some other splicing variants are expressed in embryonic skeletal muscles, although physiological functions of these variants are not clear.
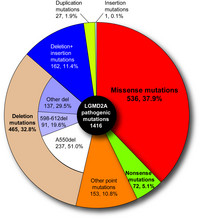
In 1995, mutations in CAPN3 were revealed to be responsible for limb-girdle muscular dystrophy type 2A (LGMD2A) (Richard et al. 1995). Positions of the mutation fund in the LGMD2A patients distribute widely in CAPN3, more than half of which are missense mutations. No ''hot point'' was found, making its diagnosis very difficult (Fig. 7). A primary cause of LGMD2A is a defect of protease activity, not structural property, of p94 (Ono et al. 1998).
- nCL-2 and nCL-2’ (CAPN8)
nCL-2 and nCL-2’ are alternative splicing products of CAPN8, respectively, with and without C2-like and 5-EF-hand domains (Hata et al. 2001; Sorimachi et al. 1993a). They are predominantly expressed in the stomach. nCL-2 is highly similar to mCL/CAPN2 along whole molecule (ca. 62 % identical). Moreover, CAPN8 and CAPN2 are closely located, and their transcripts have overlap, i.e., complementary sequences. Recombinant nCL-2 and nCL-2’ proteins can be expressed in E. coli, and showed Ca2+-dependent caseinolytic activities. X. laevis also has nCL-2 orthologue, xCL-2, and disruption of xCL-2 causes severe developmental defect (Cao et al. 2001).
- nCL-4/CAPN9
nCL-4/CAPN9 is a typical calpain homologue whose expression predominates in digestive tracts if not none in other organs (Lee et al. 1998). It has overall similarity to μCL/CAPN1 and mCL/CAPN2, and requires 30K/CAPNS1 for its activity (Lee et al. 1999). Recombinant human nCL-4+30K protein showed Ca2+-dependent caseinolytic activity, which was inhibited by calpastatin and other cysteine protease inhibitors as in the case of conventional calpains. Involvement of nCL-4 in anti-tumorigenesis was reported in human gastric cancer and NIH3T3 transformation (Liu et al. 2000; Yoshikawa et al. 2000).
- Skeletal muscle-specific calpain, p94/CAPN3
- TRA-3 and its orthologues [TRA-3 subfamily]
TRA-3 is involved in sex determination cascade of C. elegans (Barnes & Hodgkin 1996; Sokol & Kuwabara 2000). Although enzymatic characterization of purified enzyme has not yet been reported, protease activity of TRA-3 is Ca2+-dependent and necessary for female development in XX hermaphrodites by processing TRA-2A membrane protein. Recently, TRA-3 has been reported to be involved in necrotic neuronal cell death in cooperation with cathepsins and in temperature-dependent body size determination (Kammenga et al. 2007; Syntichaki et al. 2002). Mammals have two orthologues of TRA-3, i.e., hTRA-3/CAPN5, and CAPN6, whose amino acid sequences are more than 30 % identical to that of TRA-3 (Dear et al. 1997; Mugita et al. 1997). The domain T, which is conserved in all three of these molecules, has weak similarity to C2-domain. Surprisingly, CAPN6 apparently has no active site residues (active site Cys is substituted with Lys), strongly suggesting that CAPN6 has no proteolytic activity. Recently, CAPN6 has been reported to play a role on regulation of microtubule network (Tonami et al. 2007).
- Cpl1p, PalB and its orthologues [PalB subfamily]
Cpl1p is the only calpain homologue found in S. cerevisiae, and is considered both structurally and functionally to be the orthologue of Aspergillus PalB, which plays important roles in adaptation of fungi to alkaline conditions (Denison et al. 1995; Futai et al. 1999). CPL1, also called RIM13, is involved in both alkaline adaptation and sporulation process of yeast through its processing activity. Rim101p and the Aspergillus orthologue, PacC are probable in vivo substrates for Cpl1p and PalB, respectively (Diez et al. 2002; Hayashi et al. 2005; Herranz et al. 2005; Penas et al. 2007). Several other yeasts also have a Cpl1p orthologue. Cpl1p, PalB, and their orthologues share somewhat conserved domains in the N- and C-terminal regions: in the N-terminus, there is a MIT domain, which is involved in microtubule interaction and trafficking; moderately and highly diverged C2-like domains are in the C-terminal region, latter of which was also called PalB homology domain (PBH). Mammals have one orthologue, PalBH/CAPN7, whose physiological functions are unknown.
Although there is no MIT domain at the N-terminus, CAPN10 also belongs to the PalB subfamily in that it has two C2-like domains; one is moderately conserved, but the other is rather diverged. This homologue was identified by reverse genetics of non insulin-dependent diabetes mellitus (NIDDM, type 2 diabetes) (Horikawa et al. 2000). The single nucleotide polymorphism (SNP) in the intron 3 of CAPN10 is statistically related to a risk of NIDDM. This SNP probably affects transcription levels of CAPN10. CAPN10 generates several alternative splicing products. Physiological functions of calpain 10/CAPN10 are unclear.
-
SOL and its orthologues [SOL sub-family]
The drosophila gene responsible for a defect in neuronal cells (small optic lobes) was positionally cloned, showing to encode a calpain homologue with several Zn-finger motifs at the N-terminus (Delaney et al. 1991). Mammals have one orthologue, SOLH/CAPN15, and C. elegans has several. They share conserved C-terminal structure called SOL homology domain (SOH).
- DEK1 and its orthologues [DEK1 subfamily]
Maize defective kernel 1 gene required for aleurone cell development in the endosperm of maize grains revealed that it encodes plant calpain homologue with 21 trans-membrane regions at the N-terminus and C2-like domain, significantly similar to domain III, at the C-terminus (Lid et al. 2002). Rice, Arabidopsis, and loblolly pine have very conserved orthologues (above 70 % identity), and the whole genome sequence of Arabidopsis predicts DEK1 is the only calpain homologue in the genome. Structure and functions of DEK1 subfamily members are quite intriguing, and the studies have just begun.
- Other calpain homologues
Drosophila also has two homologous typical calpains, CALPA and CALPB (Emori & Saigo 1994; Jekely & Friedrich 1999; Theopold et al. 1995) (see Fig. 1). Both have essentially the same domain structures as human μCL and mCL, but CALPA has a small insertion sequence (DIS) between EF-1 and EF-2, and CALPB has a rather long domain I. Both drosophila calpains are active without a small subunit, and Ca2+ concentrations for half-maximal activity are 2.2mM and 3.3mM for CALPA and CALPB, respectively (Jekely & Friedrich 1999). Since CALPA and CALPB are equally similar to μCL and mCL, it is impossible to conclude which drosophila calpain corresponds to μ-calpain.
Schistosomes, Schistosoma mansoni and Schistosoma japonicum, have at least one typical calpain homologue (tentatively called Sm- and Sj-calpains, respectively) (Andresen et al. 1991; Karcz et al. 1991; Scott & McManus 2000; Zhang et al. 2000). Sm- and Sj-calpains are among the most immunogenic components of schistosomes, and, thus, are very important targets for vaccination against the parasites.
As described in TRA-3 subfamily, some of calpain homologues show substituted residues in either of very conserved active site residues, Cys, His, and Asn. Besides CAPN6 and drosophila CG3692, some of C. elegans and trypanosome homologues do not have one or more of the active site residues (Ersfeld et al. 2005). These molecules are supposed not to have Cys protease activity. Some of C. elegans calpain homologues have Gly-rich sequence, which is characteristic to domain V of calpain regulatory subunits (CAPNS1 and CAPNS2). Physiological significance of these structural features have yet to be discovered.
|
Fig. 7. Pathogenic mutations found in limb-girdle muscular dystrophy type 2A patients Close to 40% of all are missense mutations, and more than half are point mutations. |
- Andresen, K., Tom, T.D. & Strand, M. (1991) Characterization of cDNA clones encoding a novel calcium-activated neutral proteinase from Schistosoma mansoni. J Biol Chem 266, 15085-15090.
- Arthur, J.S., Elce, J.S., Hegadorn, C., Williams, K. & Greer, P.A. (2000) Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol. Cell Biol. 20, 4474-4481.
- Azam, M., Andrabi, S.S., Sahr, K.E., Kamath, L., Kuliopulos, A. & Chishti, A.H. (2001) Disruption of the mouse mu-calpain gene reveals an essential role in platelet function. Mol. Cell. Biol. 21, 2213-2220.
- Barnes, T.M. & Hodgkin, J. (1996) The tra-3 sex determination gene of Caenorhabditis elegans encodes a member of the calpain regulatory protease family. EMBO J. 15, 4477-4484.
- Brown, N. & Crawford, C. (1993) Structural modifications associated with the change in Ca2+ sensitivity on activation of m-calpain. FEBS Lett 322, 65-68.
- Cao, Y., Zhao, H. & Grunz, H. (2001) XCL-2 is a novel m-type calpain and disrupts morphogenetic movements during embryogenesis in Xenopus laevis. Dev. Growth Differ. 43, 563-571.
- Dayton, W.R., Goll, D.E., Zeece, M.G., Robson, R.M. & Reville, W.J. (1976) A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry 15, 2150-2158.
- Dear, N., Matena, K., Vingron, M. & Boehm, T. (1997) A new subfamily of vertebrate calpains lacking a calmodulin-like domain: implications for calpain regulation and evolution. Genomics 45, 175-184.
- Delaney, S.J., Hayward, D.C., Barleben, F., Fischbach, K.F. & Miklos, G.L. (1991) Molecular cloning and analysis of small optic lobes, a structural brain gene of Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 88, 7214-7218.
- Denison, S.H., Orejas, M. & Arst, H.N., Jr. (1995) Signaling of ambient pH in Aspergillus involves a cysteine protease. J. Biol. Chem. 270, 28519-28522.
- Diez, E., Alvaro, J., Espeso, E.A., et al. (2002) Activation of the Aspergillus PacC zinc finger transcription factor requires two proteolytic steps. EMBO J. 21, 1350-1359.
- Drummond, G.I. & Duncan, L. (1968) On the mechanism of activation of phosphorylase b kinase by calcium. J Biol Chem 243, 5532-5538.
- Dutt, P., Croall, D.E., Arthur, S.C., et al. (2006) m-Calpain is required for preimplantation embryonic development in mice. BMC Dev. Biol. 6, DOI:10.1186/1471-1213X-1186-1183.
- Emori, Y. & Saigo, K. (1994) Calpain localization changes in coordination with actin-related cytoskeletal changes during early embryonic development of Drosophila. J Biol Chem 269, 25137-25142.
- Ersfeld, K., Barraclough, H. & Gull, K. (2005) Evolutionary relationships and protein domain architecture in an expanded calpain superfamily in kinetoplastid parasites. J Mol Evol 61, 742-757.
- Futai, E., Maeda, T., Sorimachi, H., Kitamoto, K., Ishiura, S. & Suzuki, K. (1999) The protease activity of a calpain-like cysteine protease in Saccharomyces cerevisiae is required for alkaline adaptation and sporulation. Mol. Gen. Genet. 260, 559-568.
- Goll, D.E., Thompson, V.F., Li, H., Wei, W. & Cong, J. (2003) The calpain system. Physiol. Rev. 83, 731-801.
- Guroff, G. (1964) A neutral calcium-activated proteinase from the soluble fraction of rat brain. J. Biol. Chem. 239, 149-155.
- Hata, S., Nishi, K., Kawamoto, T., et al. (2001) Both the conserved and the unique gene structure of stomach-specific calpains reveal processes of calpain gene evolution. J Mol Evol 53, 191-203.
- Hayashi, M., Fukuzawa, T., Sorimachi, H. & Maeda, T. (2005) Constitutive activation of the pH-responsive Rim101 pathway in yeast mutants defective in late steps of the MVB/ESCRT pathway. Mol. Cell. Biol. 25, 9478-9490.
- Herranz, S., Rodriguez, J.M., Bussink, H.J., et al. (2005) Arrestin-related proteins mediate pH signaling in fungi. Proc. Natl. Acad. Sci. U.S.A. 102, 12141-12146.
- Horikawa, Y., Oda, N., Cox, N.J., et al. (2000) Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat. Genet. 26, 163-175.
- Huston, R.B. & Krebs, E.G. (1968) Activation of skeletal muscle phosphorylase kinase by Ca2+. II. Identification of the kinase activating factor as a proteolytic enzyme. Biochemistry 7, 2116-2122.
- Ishiura, S., Murofushi, H., Suzuki, K. & Imahori, K. (1978) Studies of a calcium-activated neutral protease from chicken skeletal muscle. I. Purification and characterization. J. Biochem. (Tokyo) 84, 225-230.
- Jekely, G. & Friedrich, P. (1999) Characterization of two recombinant Drosophila calpains. CALPA and a novel homolog, CALPB. J Biol Chem 274, 23893-23900.
- Kammenga, J.E., Doroszuk, A., Riksen, J.A., et al. (2007) A Caenorhabditis elegans Wild Type Defies the Temperature-Size Rule Owing to a Single Nucleotide Polymorphism in tra-3. PLoS Genet 3, e34.
- Karcz, S.R., Podesta, R.B., Siddiqui, A.A., Dekaban, G.A., Strejan, G.H. & Clarke, M.W. (1991) Molecular cloning and sequence analysis of a calcium-activated neutral protease (calpain) from Schistosoma mansoni. Mol Biochem Parasitol 49, 333-336.
- Lee, H.J., Sorimachi, H., Jeong, S.Y., Ishiura, S. & Suzuki, K. (1998) Molecular cloning and characterization of a novel tissue-specific calpain predominantly expressed in the digestive tract. Biol. Chem. 379, 175-183.
- Lee, H.J., Tomioka, S., Kinbara, K., et al. (1999) Characterization of a human digestive tract-specific calpain, nCL-4, expressed in the baculovirus system. Arch. Biochem. Biophys. 362, 22-31.
- Lid, S.E., Gruis, D., Jung, R., et al. (2002) The defective kernel 1 (dek1) gene required for aleurone cell development in the endosperm of maize grains encodes a membrane protein of the calpain gene superfamily. Proc. Natl. Acad. Sci. U.S.A. 99, 5460-5465.
- Liu, K., Li, L. & Cohen, S.N. (2000) Antisense RNA-mediated deficiency of the calpain protease, nCL-4, in NIH3T3 cells is associated with neoplastic transformation and tumorigenesis. J Biol Chem 275, 31093-31098.
- Meyer, W.L., Fischer, E.H. & Krebs, E.G. (1964) Activation of skeletal muscle phosphorylase kinase by Ca2+. Biochemistry 3, 1033-1039.
- Mugita, N., Kimura, Y., Ogawa, M., Saya, H. & Nakao, M. (1997) Identification of a novel, tissue-specific calpain htra-3; a human homologue of the Caenorhabditis elegans sex determination gene. Biochem Biophys Res Commun 239, 845-850.
- Ohno, S., Emori, Y., Imajoh, S., Kawasaki, H., Kisaragi, M. & Suzuki, K. (1984) Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature 312, 566-570.
- Ono, Y., Shimada, H., Sorimachi, H., et al. (1998) Functional defects of a muscle-specific calpain, p94, caused by mutations associated with limb-girdle muscular dystrophy type 2A. J. Biol. Chem. 273, 17073-17078.
- Ono, Y., Sorimachi, H. & Suzuki, K. (1999) New aspect of the research on limb-girdle muscular dystrophy 2A: a molecular biologic and biochemical approach to pathology. Trends Cardiovasc Med 9, 114-118.
- Ono, Y., Torii, F., Ojima, K., et al. (2006) Suppressed disassembly of autolyzing p94/CAPN3 by N2A connectin/titin in a genetic reporter system. J. Biol. Chem. 281, 18519-18531.
- Penas, M.M., Hervas-Aguilar, A., Munera-Huertas, T., et al. (2007) Further characterization of the signaling proteolysis step in the Aspergillus nidulans pH signal transduction pathway. Eukaryot. Cell, in press.
- Richard, I., Broux, O., Allamand, V., et al. (1995) Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 81, 27-40.
- Scott, J.C. & McManus, D.P. (2000) Characterisation and expression of a cDNA encoding the 80-kDa large subunit of Schistosoma japonicum calpain. Parasitol Int 48, 205-214.
- Sokol, S.B. & Kuwabara, P.E. (2000) Proteolysis in Caenorhabditis elegans sex determination: cleavage of TRA-2A by TRA-3. Genes Dev 14, 901-906.
- Sorimachi, H., Imajoh-Ohmi, S., Emori, Y., et al. (1989) Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m- and mu-types. Specific expression of the mRNA in skeletal muscle. J. Biol. Chem. 264, 20106-20111.
- Sorimachi, H., Ishiura, S. & Suzuki, K. (1993a) A novel tissue-specific calpain species expressed predominantly in the stomach comprises two alternative splicing products with and without Ca2+-binding domain. J. Biol. Chem. 268, 19476-19482.
- Sorimachi, H., Ishiura, S. & Suzuki, K. (1997) Structure and physiological function of calpains. Biochem J 328 ( Pt 3), 721-732.
- Sorimachi, H. & Suzuki, K. (2001) The structure of calpain. J. Biochem. 129, 653-664.
- Sorimachi, H., Toyama-Sorimachi, N., Saido, T.C., et al. (1993b) Muscle-specific calpain, p94, is degraded by autolysis immediately after translation, resulting in disappearance from muscle. J. Biol. Chem. 268, 10593-10605.
- Sorimachi, H., Tsukahara, T., Okada-Ban, M., Sugita, H., Ishiura, S. & Suzuki, K. (1995) Identification of a third ubiquitous calpain species--chicken muscle expresses four distinct calpains. Biochim Biophys Acta 1261, 381-393.
- Stabach, P.R., Cianci, C.D., Glantz, S.B., Zhang, Z. & Morrow, J.S. (1997) Site-directed mutagenesis of alpha II spectrin at codon 1175 modulates its mu-calpain susceptibility. Biochemistry 36, 57-65.
- Suzuki, K. (1991) Nomenclature of calcium dependent proteinase. Biomed Biochim Acta 50, 483-484.
- Suzuki, K., Hata, S., Kawabata, Y. & Sorimachi, H. (2004) Structure, activation, and biology of calpain. Diabetes 53, S12-18.
- Syntichaki, P., Xu, K., Driscoll, M. & Tavernarakis, N. (2002) Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature 419, 939-944.
- Theopold, U., Pinter, M., Daffre, S., et al. (1995) CalpA, a Drosophila calpain homolog specifically expressed in a small set of nerve, midgut, and blood cells. Mol Cell Biol 15, 824-834.
- Tompa, P., Buzder-Lantos, P., Tantos, A., et al. (2004) On the sequential determinants of calpain cleavage. J Biol Chem 279, 20775-20785.
- Tonami, K., Kurihara, Y., Aburatani, H., Uchijima, Y., Asano, T. & Kurihara, H. (2007) Calpain 6 Is Involved in Microtubule Stabilization and Cytoskeletal Organization. Mol Cell Biol.
- Yoshikawa, Y., Mukai, H., Hino, F., Asada, K. & Kato, I. (2000) Isolation of two novel genes, down-regulated in gastric cancer. Jpn J Cancer Res 91, 459-463.
- Zhang, R., Suzuki, T., Takahashi, S., et al. (2000) Cloning and molecular characterization of calpain, a calcium-activated neutral proteinase, from different strains of Schistosoma japonicum. Parasitol Int 48, 232-242.
- Zimmerman, U.J., Boring, L., Pak, J.H., Mukerjee, N. & Wang, K.K. (2000) The calpain small subunit gene is essential: its inactivation results in embryonic lethality. IUBMB Life 50, 63-68.
common Ca2+-binding structure composed of 8 anti-parallel β-strand structure
calpain homepageCalpain related reagents are now available from several companies, which is summarized in the calpain homepage (http://ag.arizona.edu/calpains/reagents.html).
cysteine proteasepeptide bond hydrolyzing enzyme whose active site is composed of Cys residue
EF-hand motifcommon Ca2+-binding motif composed of 2 α-helices (E- and F-helices) and Ca2+-binding loop in between LGMD2A Information on pathogenic mutations of LGMD2A is available in the Human Gene Mutation Database (http://archive.uwcm.ac.uk/uwcm/mg/search/119751.html)
muscular dystrophyprogressive deterioration of muscle tissue and resultant weakness caused by a defect of number of muscle genes such as dystrophin, sarcoglycan, merosin, laminin, and calpain