XSB0443 : BCL2-associated X protein isoform beta [Homo sapiens]
[ CaMP Format ]
This entry is computationally expanded from SB0026
* Basic Information
| Organism | Homo sapiens (human) |
| Protein Names | Apoptosis regulator BAX; Bcl-2-like protein 4; Bcl2-L-4; BCL2-associated X protein isoform beta |
| Gene Names | BAX; BCL2L4; BCL2-associated X protein |
| Gene Locus | 19q13.3-q13.4; chromosome 19 |
| GO Function | Not available |
* Information From OMIM
Description: The proapoptotic BAX protein induces cell death by acting on mitochondria.
Function: Development as well as maintenance of many adult tissues is achieved by several dynamically regulated processes that include cell proliferation, differentiation, and programmed cell death. Oltvai et al. (1993) noted that, in the latter process, cells are eliminated by a highly characteristic suicide program called apoptosis. The best-defined genetic pathway of cell death exists in the nematode Caenorhabditis elegans. Two autosomal recessive death effector genes, ced-3 and ced-4, are required for the death of all 131 cells destined to die during worm development. One autosomal dominant death repressor gene, ced-9, can save those cells in its gain-of-function form. This implies that both effector and repressor genes also exist within each mammalian cell death pathway. BCL2 is one such mammalian gene that has been identified; it functions as a repressor of programmed cell death.
Function: Oltvai et al. (1993) showed that BCL2 associates in vivo with a 21-kD program partner, BAX. BAX shows extensive amino acid homology with BCL2 and forms homodimers and heterodimers with BCL2 in vivo. When BAX predominates, programmed cell death is accelerated, and the death repressor activity of BCL2 is countered. Their findings suggested to Oltvai et al. (1993) a model in which the ratio of BCL2 to BAX determines survival or death following an apoptotic stimulus.
Function: The BAX gene promoter region contains 4 motifs with homology to consensus p53-binding sites. In cotransfection assays using p53-deficient tumor cell lines, Miyashita and Reed (1995) found that wildtype but not mutant p53 expression plasmids transactivated a reporter gene plasmid that utilized the BAX gene promoter to drive transcription of chloramphenicol acetyltransferase. Introduction of mutations into the consensus p53-binding site sequences abolished p53 responsiveness of the reporter gene plasmids. Taken together, the results suggested that BAX is a primary-response gene for p53 (OMIM:191170) and is involved in a p53-regulated pathway for induction of apoptosis.
Function: Apte et al. (1995) isolated a BAX cDNA clone in which the mRNA encoded by exon 3 was absent. The skipping of exon 3 predicted the existence of an interstitially truncated form of the major BAX protein (BAX-alpha), termed BAX-delta. Unlike 2 previously described variant forms, BAX-delta retains the functionally critical C-terminal membrane anchor region, as well as the BCL2 homology 1 and 2 (BH1 and BH2) domains.
Function: Cartron et al. (2002) examined the expression of BAX in 55 patients with glioblastoma multiforme (see OMIM:137800), the most common and aggressive form of brain tumors. The authors identified a novel form of BAX, designated BAX-psi, which was present in 24% of the patients. BAX-psi is an N-terminal truncated form of BAX which results from a partial deletion of exon 1 of the BAX gene. BAX-psi and the wildtype form, BAX-alpha, are encoded by distinct mRNAs, both of which are present in normal tissues. Glial tumors expressed either BAX-alpha or BAX-psi proteins, an apparent consequence of an exclusive transcription of the corresponding mRNAs. The BAX-psi protein was preferentially localized to mitochondria and was a more powerful inducer of apoptosis than BAX-alpha. BAX-psi tumors exhibited slower proliferation in Swiss nude mice, and this feature could be circumvented by the coexpression of the BCL2 (OMIM:151430) transgene, the functional antagonist of BAX. The expression of BAX-psi correlated with a longer survival in patients (18 months versus 10 months for BAX-alpha patients). The authors hypothesized a beneficial involvement of the psi variant of BAX in tumor progression.
Function: During transduction of an apoptotic signal into the cell, there is an alteration in the permeability of the membranes of the cell's mitochondria, which causes the translocation of the apoptogenic protein cytochrome c into the cytoplasm, which in turn activates death-driving proteolytic proteins known as caspases (see OMIM:147678). The BCL2 family of proteins, whose members may be antiapoptotic or proapoptotic, regulates cell death by controlling this mitochondrial membrane permeability during apoptosis. Shimizu et al. (1999) created liposomes that carried the mitochondrial porin channel VDAC (OMIM:604492) to show that the recombinant proapoptotic proteins Bax and Bak (OMIM:600516) accelerate the opening of VDAC, whereas the antiapoptotic protein BCLXL (OMIM:600039) closes VDAC by binding to it directly. Bax and Bak allow cytochrome c to pass through VDAC out of liposomes, but passage is prevented by BCLXL. In agreement with this, VDAC1-deficient mitochondria from a mutant yeast did not exhibit a Bax/Bak-induced loss in membrane potential and cytochrome c release, both of which were inhibited by BCLXL. Shimizu et al. (1999) concluded that the BCL2 family of proteins bind to the VDAC in order to regulate the mitochondrial membrane potential and the release of cytochrome c during apoptosis.
Function: Since the BAX protein regulates apoptosis in a cellular pathway that involves both BCL2 and p53, 2 molecules associated with human glioma agenesis, Chou et al. (1996) evaluated the possibility that BAX functions as a glioma tumor suppressor gene. Somatic cell hybrid panels, fluorescence in situ hybridization, and cosmid mapping localized the BAX gene to 19q13.3 at the telomeric end of the glioma candidate region frequently deleted in gliomas. However, routine and pulsed field gel electrophoresis/Southern blotting studies failed to reveal large scale deletions or rearrangements of the BAX gene in gliomas. In addition, SSCP analysis of 6 BAX exons and flanking intronic sequences did not disclose mutations in 20 gliomas with allelic loss of the other copy of 19q. Thus, BAX is probably not the 19q glioma tumor suppressor gene.
Function: To assess the role of BAX in drug-induced apoptosis in human colorectal cancer cells (HCT116 cells), Zhang et al. (2000) generated cells that lacked functional BAX genes. Such cells were partially resistant to the apoptotic effects of the chemotherapeutic agent 5-fluorouracil, but apoptosis was not abolished. In contrast, the absence of BAX completely abolished the apoptotic response to the chemopreventive agent sulindac and other nonsteroidal antiinflammatory drugs (NSAIDs). NSAIDs inhibited the expression of the antiapoptotic protein BCLXL, resulting in an altered ratio of BAX to BCLXL and subsequent mitochondria-mediated cell death. Zhang et al. (2000) concluded that their results establish an unambiguous role for BAX in apoptotic processes in human epithelial cancers and may have implications for cancer chemoprevention strategies.
Function: Studies of Bax-deficient mice indicated that the proapoptotic BAX molecule can function as a tumor suppressor. For that reason, Meijerink et al. (1998) examined human hematopoietic malignancies and found that approximately 21% of lines possessed mutations in BAX, perhaps most commonly in the acute lymphoblastic leukemia subset. Approximately half were nucleotide insertions or deletions within a deoxyguanosine (G8) tract, resulting in a proximal frameshift and loss of immunodetectable BAX protein. Other BAX mutants bore single amino acid substitutions within BH1 or BH3 domains, demonstrated altered patterns of protein dimerization, and had lost death-promoting activity.
Function: The proapoptotic BAX protein induces cell death by acting on the mitochondria. BAX binds to the permeability transition pore complex (PTPC), a composite proteaceous channel that is involved in the regulation of mitochondrial membrane permeability. Marzo et al. (1998) found that immunodepletion of Bax from PTPC or purification of PTPC from Bax-deficient mice yielded a PTPC that could not permeabilize membranes in response to atractyloside, a proapoptotic ligand of the adenine nucleotide translocator (ANT; OMIM:103220). Bax and ANT coimmunoprecipitated and interacted in the yeast 2-hybrid system. Ectopic expression of Bax induced cell death in wildtype but not in ANT-deficient yeast. Recombinant Bax and purified ANT, but neither of them alone, efficiently formed atractyloside-responsive channels in artificial membranes. Hence, the proapoptotic molecule Bax and the constitutive mitochondrial protein ANT cooperate within the PTPC to increase mitochondrial membrane permeability and to trigger cell death.
Function: The caspase-activated form of BID (OMIM:601997), tBID, triggers the homooligomerization of multidomain conserved proapoptotic family members BAK or BAX, resulting in the release of cytochrome c from mitochondria. Wei et al. (2001) found that cells lacking both BAK and BAX, but not cells lacking only one of these components, are completely resistant to tBID-induced cytochrome c release and apoptosis. Moreover, doubly deficient cells are resistant to multiple apoptotic stimuli that act through disruption of mitochondrial function: staurosporine, ultraviolet radiation, growth factor deprivation, etoposide, and the endoplasmic reticulum stress stimuli thapsigargin and tunicamycin. Thus, Wei et al. (2001) concluded that activation of a 'multidomain' proapoptotic member, BAK or BAX, appears to be an essential gateway to mitochondrial dysfunction required for cell death in response to diverse stimuli.
Function: Polycyclic aromatic hydrocarbons (PAHs) are toxic chemicals released into the environment by fossil fuel combustion. Oocyte destruction and ovarian failure occur in PAH-treated mice, and cigarette smoking causes early menopause in women. In many cells, PAHs activate the aromatic hydrocarbon receptor (AHR; OMIM:600253), a member of the Per-Arnt-Sim family of transcription factors. The AHR is also activated by dioxin, one of the most intensively studied environmental contaminants. Matikainen et al. (2001) demonstrated that exposure of mice to PAHs induces the expression of Bax in oocytes, followed by apoptosis. Ovarian damage caused by PAHs is prevented by Ahr or Bax inactivation. Oocytes microinjected with a Bax promoter-reporter construct show Ahr-dependent transcriptional activation after PAH, but not dioxin, treatment, consistent with findings that dioxin is not cytotoxic to oocytes. This difference in the action of PAHs versus dioxin is conveyed by a single basepair flanking each Ahr response element in the Bax promoter. Oocytes in human ovarian biopsies grafted into immunodeficient mice also accumulated Bax and underwent apoptosis after PAH exposure in vivo. Thus, Matikainen et al. (2001) concluded that AHR-driven BAX transcription is a novel and evolutionarily conserved cell-death signaling pathway responsible for environmental toxicant-induced ovarian failure.
Function: To investigate the relationship between apoptosis and the BCL2/BAX system in the human corpus luteum, Sugino et al. (2000) examined the frequency of apoptosis and expression of BCL2 and BAX in the corpus luteum during the menstrual cycle and in early pregnancy. Immunohistochemistry revealed BCL2 expression in the luteal cells in the midluteal phase and early pregnancy, but not in the regressing corpus luteum. In contrast, BAX immunostaining was observed in the regressing corpus luteum, but not in the midluteal phase or early pregnancy. The BCL2 mRNA levels in the corpus luteum during the menstrual cycle were highest in the midluteal phase and lowest in the regressing corpus luteum. In the corpus luteum of early pregnancy, BCL2 mRNA levels were significantly higher than those in the midluteal phase. In contrast, BAX mRNA levels were highest in the regressing corpus luteum and remarkably low in the corpus luteum of early pregnancy. When corpora lutea of the midluteal phase were incubated with CG (see OMIM:118850), CG significantly increased the mRNA and protein levels of BCL2 and significantly decreased those of BAX. Sugino et al. (2000) concluded that BCL2 and BAX may play important roles in the regulation of the life span of the human corpus luteum by controlling the rate of apoptosis. CG may act to prolong the life span of the corpus luteum by increasing BCL2 expression and decreasing BAX expression when pregnancy occurs.
Function: Li et al. (2001) found increased levels of BAX and its mRNA in the stroma but not in the endothelium of Fuchs dystrophy (OMIM:610158) corneas. Following exposure to camptothecin (a DNA synthesis inhibitor known to induce apoptosis in vitro), keratocytes from patients produced an increased level of BAX and a low level of BCL2 distinctly different from the response of normal keratocytes. The authors concluded that their results point to a disease-related disturbance in the regulation of apoptosis in Fuchs dystrophy. They proposed that excessive apoptosis might be an important mechanism in the pathogenesis of Fuchs dystrophy.
Function: Vaskivuo et al. (2001) investigated the extent and localization of apoptosis in human fetal (aged 13 to 40 weeks) and adult ovaries. They also studied the expression of apoptosis-regulating proteins BCL2 and BAX. Expression of BCL2 was observed only in the youngest fetal ovaries (weeks 13 to 14), and BAX was present in the ovaries throughout the entire fetal period. In adult ovaries, apoptosis was detected in granulosa cells of secondary and antral follicles, and BCL2 and BAX were expressed from primary follicles onwards. Apoptosis was found in ovarian follicles throughout fetal and adult life. During fetal development, apoptosis was localized mainly to primary oocytes and was highest between weeks 14 and 28, decreasing thereafter toward term.
Function: LeBlanc et al. (2002) demonstrated that BAX can be essential for death receptor-mediated apoptosis in cancer cells. BAX-deficient human colon carcinoma cells were resistant to death-receptor ligands, whereas BAX-expressing sister clones were sensitive. BAX was dispensable for apical death-receptor signaling events including caspase-8 (OMIM:601763) activation, but crucial for mitochondrial changes and downstream caspase activation. Treatment of colon cancer cells deficient in DNA mismatch repair with the TRAIL (OMIM:603598) selected in vitro or in vivo for refractory subclones with BAX frameshift mutations including deletions at a novel site. Chemotherapeutic agents upregulated expression of the TRAIL receptor DR5 (OMIM:603612) and the BAX homolog BAK (OMIM:600516) in BAX -/- cells, and restored TRAIL sensitivity in vitro and in vivo. Thus, LeBlanc et al. (2002) concluded that BAX mutation in mismatch repair-deficient tumors can cause resistance to death receptor-targeted therapy, but pre-exposure to chemotherapy rescues tumor sensitivity.
Function: Guo et al. (2003) found that Bax coimmunoprecipitated with humanin (OMIM:606120), a peptide with neuroprotective activities against Alzheimer disease (OMIM:104300)-associated insults, and that humanin rescued rat hippocampal neurons from Bax-induced lethality. Humanin prevented the translocation of Bax from the cytosol to the mitochondria and suppressed cytochrome c release. Guo et al. (2003) noted that the predicted humanin peptides from the nuclear-encoded peptide and the mitochondrial-encoded peptide were both able to bind Bax and prevent apoptosis. The authors suggested that the HN gene arose from mitochondria and transferred to the nuclear genome, providing a protective mechanism for additional organelles.
Function: Chipuk et al. (2004) found that cytosolic localization of endogenous wildtype or trans-activation-deficient p53 (OMIM:191170) was necessary and sufficient for apoptosis. p53 directly activated the proapoptotic BCL2 protein BAX in the absence of other proteins to permeabilize mitochondria and engage the apoptotic program. p53 also released both proapoptotic multidomain proteins and BH3-only proteins that were sequestered by BCL-XL (see OMIM:600039). The transcription-independent activation of BAX by p53 occurred with similar kinetics and concentrations to those produced by activated BID (OMIM:601997). Chipuk et al. (2004) proposed that when p53 accumulates in the cytosol, it can function analogously to the BH3-only subset of proapoptotic BCL2 proteins to activate BAX and trigger apoptosis.
Function: Clusterin (CLU; OMIM:185430) is overexpressed in human prostate and breast cancers and in squamous cell carcinomas, and suppression of CLU renders these cells sensitive to chemotherapeutic drug-mediated apoptosis. Zhang et al. (2005) found that intracellular CLU inhibited apoptosis by interfering with BAX activation in mitochondria. CLU specifically interacted with BAX that was conformationally altered by chemotherapeutic drugs, and the interaction inhibited BAX-mediated apoptosis. Zhang et al. (2005) concluded that elevated CLU levels in human cancers may promote oncogenic transformation and tumor progression by interfering with BAX proapoptotic activities.
Function: Perier et al. (2005) presented evidence suggesting that mitochondrial complex I deficiency (OMIM:252010) does not autonomously kill cells but rather sensitizes neurons to the action of Bax through mitochondrial oxidative damage. In isolated brain cell mitochondria, inhibition of complex I activity resulted in increased levels of reactive oxygen species and promoted Bax-dependent cytochrome c release. Perier et al. (2005) proposed a model in which complex I defects lower the threshold for activation of mitochondrial-dependent apoptosis by Bax, thus rendering compromised neurons more prone to degeneration.
Function: Hetz et al. (2006) investigated the unfolded protein response signaling events in mice in the absence of proapoptotic BCL2 family members Bax and Bak (OMIM:600516) using double-knockout mice. Double-knockout mice responded abnormally to tunicamycin-induced endoplasmic reticulum (ER) stress in the liver, with extensive tissue damage and decreased expression of the IRE1 substrate X box-binding protein-1 (Xbp1; OMIM:194355) and its target genes. ER-stressed double knockout cells showed deficient IRE1-alpha (OMIM:604033) signaling. BAX and BAK formed a protein complex with the cytosolic domain of IRE1-alpha that was essential for IRE1-alpha activation. Thus, Hetz et al. (2006) concluded that BAX and BAK function at the ER membrane to activate IRE1-alpha signaling and to provide a physical link between members of the core apoptotic pathway and the unfolded protein response.
Function: Two members of the BCL2 family, BAX and BAK (OMIM:600516), change intracellular location early in the promotion of apoptosis to concentrate in focal clusters at sites of mitochondrial division. Karbowski et al. (2006) reported that in healthy cells, BAX or BAK is required for normal fusion of mitochondria into elongated tubules. BAX seems to induce mitochondrial fusion by activating assembly of the large GTPase MFN2 (OMIM:608507) and changing its submitochondrial distribution and membrane mobility--properties that correlate with different GTP-bound states of MFN2. Karbowski et al. (2006) concluded that BAX and BAK regulate mitochondrial dynamics in healthy cells and that BCL2 family members may also regulate apoptosis through organelle morphogenesis machineries.
Function: A central issue in the regulation of apoptosis by the BCL2 family is whether its BH3-only members initiate apoptosis by directly binding to the essential cell death mediators BAX and BAK, or whether they can act indirectly, by engaging their prosurvival BCL2-like relatives. Contrary to the direct-activation model, Willis et al. (2007) showed that BAX and BAK can mediate apoptosis without discernable association with the putative BH3-only activators (BIM, OMIM:603827; BID, OMIM:601997; and PUMA, OMIM:605854), even in cells with no BIM or BID and reduced PUMA. Willis et al. (2007) concluded that BH3-only proteins induce apoptosis at least primarily by engaging with multiple prosurvival relatives guarding BAX and BAK.
* Structure Information
1. Primary Information
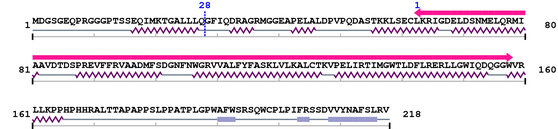
Length: 218 aa
Average Mass: 24.220 kDa
Monoisotopic Mass: 24.204 kDa
2. Domain Information
Annotated Domains: interpro / pfam / smart / prosite
Computationally Assigned Domains (Pfam+HMMER):
| domain name | begin | end | score | e-value |
|---|---|---|---|---|
| --- cleavage 28 --- | ||||
| Bcl-2 1. | 63 | 158 | 156.1 | 1.1e-43 |
3. Sequence Information
Fasta Sequence: XSB0443.fasta
Amino Acid Sequence and Secondary Structures (PsiPred):
* Cleavage Information
1 [sites]
Cleavage sites (±10aa)
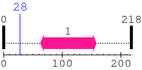
[Site 1] IMKTGALLLQ28-GFIQDRAGRM
Gln28  Gly
Gly
 |
|||||||||
| P10 | P9 | P8 | P7 | P6 | P5 | P4 | P3 | P2 | P1 |
|---|---|---|---|---|---|---|---|---|---|
| Ile19 | Met20 | Lys21 | Thr22 | Gly23 | Ala24 | Leu25 | Leu26 | Leu27 | Gln28 |
 |
|||||||||
| P1' | P2' | P3' | P4' | P5' | P6' | P7' | P8' | P9' | P10' |
| Gly29 | Phe30 | Ile31 | Gln32 | Asp33 | Arg34 | Ala35 | Gly36 | Arg37 | Met38 |
 Sequence conservation (by blast)
Sequence conservation (by blast) Sequence conservation (by blast)
Sequence conservation (by blast)
| Reference peptide (cleaved bond±30 residues) |
|---|
| MDGSGEQPRGGGPTSSEQIMKTGALLLQGFIQDRAGRMGGEAPELALDPVPQDASTKK |
Summary
| # | organism | max score | hits | top seq |
|---|---|---|---|---|
| 1 | Homo sapiens | 119.00 | 8 | BCL2-associated X protein isoform beta |
| 2 | synthetic construct | 119.00 | 2 | BCL2-associated X protein |
| 3 | Macaca mulatta | 116.00 | 2 | PREDICTED: similar to BCL2-associated X protein is |
| 4 | Pan troglodytes | 116.00 | 1 | PREDICTED: similar to Bax alpha |
| 5 | Felis catus | 113.00 | 3 | BAX |
| 6 | Bos taurus | 111.00 | 2 | BCL2-associated X protein |
| 7 | Canis lupus familiaris | 111.00 | 1 | BCL2-associated X protein |
| 8 | N/A | 99.00 | 2 | B |
| 9 | Mus musculus | 96.70 | 4 | Bcl2-associated X protein |
| 10 | Cricetulus griseus | 95.90 | 1 | Bax |
| 11 | Rattus norvegicus | 94.40 | 4 | bcl2-associated X protein |
| 12 | Monodelphis domestica | 90.90 | 1 | PREDICTED: similar to Bax alpha |
| 13 | Ovis aries | 54.30 | 1 | AF163774_1 Bcl2-associated protein Bax |
| 14 | Sus scrofa | 54.30 | 1 | Bax-alpha protein |
| 15 | Pan paniscus | 48.10 | 1 | BAX |
| 16 | Lemur catta | 45.10 | 1 | BAX |
| 17 | Rattus sp. | 35.80 | 1 | bax gb |
| 18 | Xenopus tropicalis | 35.00 | 1 | BCL2-associated X protein |
Top-ranked sequences
| organism | matching |
|---|---|
| Homo sapiens | Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 ||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 |
| synthetic construct | Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 ||||||||||||||||||||||||||||#|||||||||||||||||||||||||||||| Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 |
| Macaca mulatta | Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 ||||||||||||||||||||||||||||#||||||||||||| |||||||||||||||+ Sbjct 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGETPELALDPVPQDASTKR 58 |
| Pan troglodytes | Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 ||||||||||||||||||||||||||||#|||||||||| ||||||||||||||||||| Sbjct 72 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMRGEAPELALDPVPQDASTKK 129 |
| Felis catus | Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 ||||||||||||||||||||||||||||#||||||||||||| |||||+ ||||||||| Sbjct 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGETPELALEQVPQDASTKK 58 |
| Bos taurus | Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 ||||||||||||||||||||||||||||#||||||||||||| ||| |+ ||||||||| Sbjct 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGETPELGLEQVPQDASTKK 58 |
| Canis lupus familiaris | Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 ||||||||||||||||||||||||||||#||||||||||||| ||| |+ ||||||||| Sbjct 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGETPELPLEQVPQDASTKK 58 |
| N/A | Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 ||||||| ||||||||||||||| |||#|||||||||| || ||| |+ |||||||| Sbjct 1 MDGSGEQLGGGGPTSSEQIMKTGAFLLQ#GFIQDRAGRMAGETPELTLEQPPQDASTKK 58 |
| Mus musculus | Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 ||||||| |||||||||||||| |||#|||||||||| || ||| |+ |||||||| Sbjct 1 MDGSGEQLGSGGPTSSEQIMKTGAFLLQ#GFIQDRAGRMAGETPELTLEQPPQDASTKK 58 |
| Cricetulus griseus | Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 ||||||| ||||||||||||||| |||#|||||||||| |+ ||| |+ ||| |||| Sbjct 1 MDGSGEQLGGGGPTSSEQIMKTGAFLLQ#GFIQDRAGRMAGDTPELTLEQPPQDPSTKK 58 |
| Rattus norvegicus | Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 ||||||| ||||||||| ||||| |||#||||||| || || ||| |+ |||||||| Sbjct 1 MDGSGEQLGGGGPTSSEQFMKTGAFLLQ#GFIQDRAERMAGETPELTLEQPPQDASTKK 58 |
| Monodelphis domestica | Query 1 MDGSGEQPRGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALD------PVPQDA 54 |||||||||||| |||||||+|||+|||#|||||||||+ | |||| || |+| | Sbjct 1 MDGSGEQPRGGGATSSEQIMRTGAVLLQ#GFIQDRAGRVAGGAPELLLDSMGDSAPLPSDP 60 Query 55 STKK 58 ||+ Sbjct 61 RTKR 64 |
| Ovis aries | Query 29 GFIQDRAGRMGGEAPELALDPVPQDASTKK 58 |||||| |||||| ||| |+ ||||||||| Sbjct 1 GFIQDRDGRMGGETPELGLEQVPQDASTKK 30 |
| Sus scrofa | Query 30 FIQDRAGRMGGEAPELALDPVPQDASTKK 58 |||||||||||| ||| |+ ||||||||| Sbjct 1 FIQDRAGRMGGETPELGLEQVPQDASTKK 29 |
| Pan paniscus | Query 37 RMGGEAPELALDPVPQDASTKK 58 |||||||||||||||||||||| Query 37 RMGGEAPELALDPVPQDASTKK 58 |
| Lemur catta | Query 37 RMGGEAPELALDPVPQDASTKK 58 |||||||||||+| |||||||| Sbjct 1 RMGGEAPELALEPTPQDASTKK 22 |
| Rattus sp. | Query 37 RMGGEAPELALDPVPQDASTKK 58 || || ||| |+ |||||||| Sbjct 1 RMAGETPELTLEQPPQDASTKK 22 |
| Xenopus tropicalis | Query 9 RGGGPTSSEQIMKTGALLLQ#GFIQDRAGRMGGEAPELALDP 49 | | |+|||++|| ||| #||| || | || | Sbjct 36 RSGTGVSTEQILETGELLLN#GFISDRLQNNPDVAGARALFP 76 |
* References
[PubMed ID: 19380879] Gallenne T, Gautier F, Oliver L, Hervouet E, Noel B, Hickman JA, Geneste O, Cartron PF, Vallette FM, Manon S, Juin P, Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol. 2009 Apr 20;185(2):279-90.
[PubMed ID: 19360919] ... Yao Y, Huang C, Li ZF, Wang AY, Liu LY, Zhao XG, Luo Y, Ni L, Zhang WG, Song TS, Exogenous phosphatidylethanolamine induces apoptosis of human hepatoma HepG2 cells via the bcl-2/Bax pathway. World J Gastroenterol. 2009 Apr 14;15(14):1751-8.
[PubMed ID: 19336552] ... Morton LM, Purdue MP, Zheng T, Wang SS, Armstrong B, Zhang Y, Menashe I, Chatterjee N, Davis S, Lan Q, Vajdic CM, Severson RK, Holford TR, Kricker A, Cerhan JR, Leaderer B, Grulich A, Yeager M, Cozen W, Hoar Zahm S, Chanock SJ, Rothman N, Hartge P, Risk of non-Hodgkin lymphoma associated with germline variation in genes that regulate the cell cycle, apoptosis, and lymphocyte development. Cancer Epidemiol Biomarkers Prev. 2009 Apr;18(4):1259-70. Epub 2009 Mar
[PubMed ID: 19237173] ... Hirata H, Hinoda Y, Kikuno N, Suehiro Y, Shahryari V, Ahmad AE, Tabatabai ZL, Igawa M, Dahiya R, Bcl2 -938C/A polymorphism carries increased risk of biochemical recurrence after radical prostatectomy. J Urol. 2009 Apr;181(4):1907-12. Epub 2009 Feb 23.
[PubMed ID: 19266703] ... Cosentino M, Colombo C, Mauri M, Ferrari M, Corbetta S, Marino F, Bono G, Lecchini S, Expression of apoptosis-related proteins and of mRNA for dopaminergic receptors in peripheral blood mononuclear cells from patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2009 Jan-Mar;23(1):88-90.
[PubMed ID: 8521816] ... Chittenden T, Flemington C, Houghton AB, Ebb RG, Gallo GJ, Elangovan B, Chinnadurai G, Lutz RJ, A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995 Nov 15;14(22):5589-96.
[PubMed ID: 7566098] ... Adams MD, Kerlavage AR, Fleischmann RD, Fuldner RA, Bult CJ, Lee NH, Kirkness EF, Weinstock KG, Gocayne JD, White O, et al., Initial assessment of human gene diversity and expression patterns based upon 83 million nucleotides of cDNA sequence. Nature. 1995 Sep 28;377(6547 Suppl):3-174.
[PubMed ID: 7607685] ... Apte SS, Mattei MG, Olsen BR, Mapping of the human BAX gene to chromosome 19q13.3-q13.4 and isolation of a novel alternatively spliced transcript, BAX delta. Genomics. 1995 Apr 10;26(3):592-4.
[PubMed ID: 7834749] ... Miyashita T, Reed JC, Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995 Jan 27;80(2):293-9.
[PubMed ID: 8358790] ... Oltvai ZN, Milliman CL, Korsmeyer SJ, Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609-19.